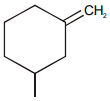
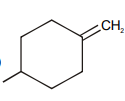
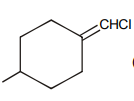
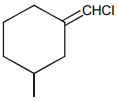
Which of the following compound requires minimum energy for free rotation across double bond between ring :
1.

2.

3.

4.





Which of the following is not nucleophile:-
1. 
2. 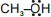
3. 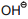
4. 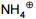
Correct order of acidic strength for following is:-
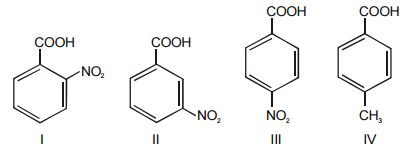
1. I > II > III > IV
2. I > III > II > IV
3. IV > III > I > II
4. III > I > II > IV
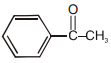
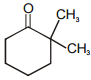
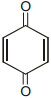
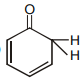
Which of the following although have -hydrogen but does not show tautomerism :-
1.
2. 
3. 
4. 
The molecule that exhibits non-planarity is:
| 1. |  |
2. |  |
| 3. |  |
4. |  |
The aromatic compound among the following is:-
| 1. | 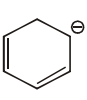 |
2. |  |
| 3. | 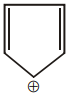 |
4. | 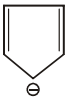 |
Which carbocation is the most stable among the options provided?
| 1. |  |
2. |  |
| 3. |  |
4. |  |
Correct stability order of the following carbocation:-
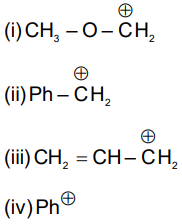
(1) ii > i > iii > iv
(2) i > ii > iii > iv
(3) iii > i > ii > iv
(4) iv > i > ii > iii
The presence of a halogen on the benzene ring in a nitration reaction leads to:
| 1. | Direct nitro group to come at meta and deactivate the ring due to –I effect of halogen. |
| 2. | Direct nitro group to come at ortho and para position and deactivate the ring due to –I effect of halogen. |
| 3. | Direct nitro group to come at meta and activate the ring toward nitration reaction. |
| 4. | Nitration reaction does not take place due to deactivation caused by –I effect of halogen. |