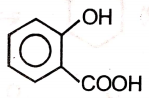
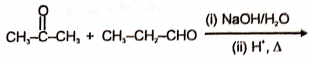
An organic compound (A) of the molecular formula gives a positive Iodoform test but does not reduce Tollen's reagent. When (A) is subjected to acid-catalyzed hydrolysis, another compound B is formed which gives positive Tollen's test. Identify the unknown compound (A).
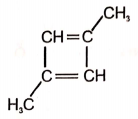
1. 
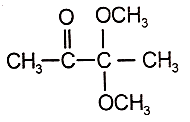
2. 
3. 
4. 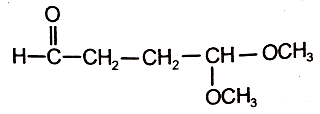



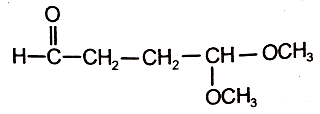
\(\mathrm{C}_2 \mathrm{H}_4 \xrightarrow{\mathrm{O}_3 / \mathrm{Zn} / \mathrm{H}_2 \mathrm{O}_2} \text { Product } \mathrm{A}\)
1. Haloform test
2. Aldol condensation
3. Cannizzaro reaction
4. Both (1) & (2)
Consider the following sequential reaction:
\(\begin{aligned} &\mathrm{RCH}=\mathrm{CH}_2 \xrightarrow[\mathrm{Zn} / \mathrm{H}_2 \mathrm{O}]{\mathrm{O}_3}(\mathrm{~A}) \xrightarrow{\mathrm{LAH}}(\mathrm{~B})+\text { (C), }\\ &\text { (B) and (C) are: } \end{aligned}\)
1. RCHO and HCHO
2. RCOOH and HCOOH
3. \(\mathrm{RCH}_3 \mathrm{OH} \text { and } \mathrm{HCOOH}\)
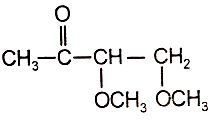
4. ![]()
The values of some of the acids are given below
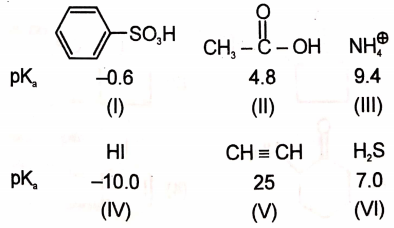
What is the correct order of leaving the ability of their conjugate bases?
1. ![]()
2. ![]()
3. ![]()
4. ![]()
Among the following esters, form a pair of least reactive and most reactive ester towards hydrolysis is-
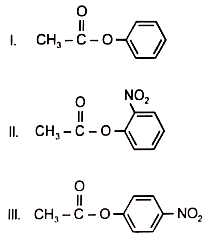
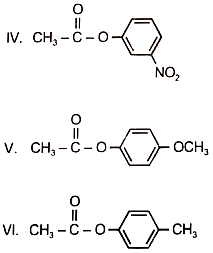
1. V. III
2. VI, II
3. I, IV
4. I, V
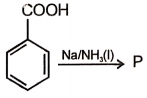
The product formed in this reaction is
1. ![]() 2.
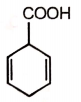
2. 
3. ![]() 4.
4. ![]()
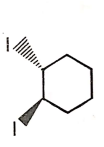
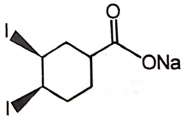
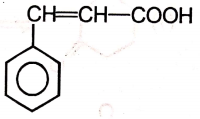
The major product of the given reaction is
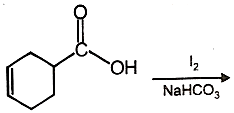
1. 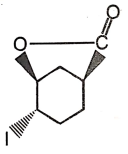 2.
2. 
3. 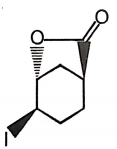 4.
4. 

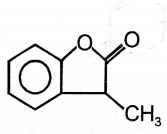 2.
2. 
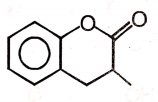 4.
4.