In the given sequence reaction which of the following is the correct structure of compounds A.

(1)

(2)

(3)

(4)






Choose the correct statement
(1) I effect transfers e– from one carbon atom to another
(2) I effect operates in both σ\p bond
(3) I effect creates not charge in molecule
(4) I effect creates partial charges and it is distance dependent
The IUPAC name of 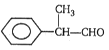 is:
is:
1. 2-Phenylpropan-3-al 2. Formylethylbenzene
3. 2-Phenylpropanal 4. Ethylformylbenzene
The IUPAC name of 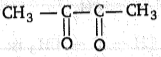 is:
is:
1. Butane-2, 3-dial
2. Butane-1, 3-dione
3. Butane-2, 3-dione
4. 1, 2-dimethylethanedione
The IUPAC name of 
1. 4-Bromo benzenamine
2. 4-Amino-1-bromobenzene
3. 4-Bromo benzenamide
4. 1-Bromo benzencarboxamide
The IUPAC name of 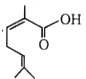 is:
is:
1. 2, 6-Dimethylhepta-2, 5-dienoic acid
2. 3, 7-Dimethylhepta-2, 5-dienoic acid
3. 1-Hydroxy-2, 6-dimethylhepta-2, 5-dienone
4. none of these
The most stable carbocation is:
1.
2.
3.
4.
The electromeric effect in organic compounds is a
1. temporary effect
2. permanent effect
3. temporary-permanent effect
4. none of the above
The stability of 2,3-dimethyl but-2-ene is more than 2-butene. This can be explained in terms of:
1. Resonance
2. Hyperconjugation
3. Electromeric effect
4. Inductive effect
(CH3)4N+ is neither an electrophile nor a nucleophile because it:
| 1. | does not have an electron pair for donation as well as cannot attract an electron pair. |
| 2. | neither has an electron pair available for donation nor can accommodate electrons since all shells of N are fully occupied. |
| 3. | can act as Lewis acid and base. |
| 4. | none of the above. |










