Select the incorrect statement regarding compounds (X), (Y) and (Z).
1. Compound (X) is and compound (Z) contains two 3C - 2e bonds
2. Compound (X) is and compound (Y) contains one 3C - 2e bonds
3. Compound (Y) is Lewis acid and stable due to back bonding
4. Compound (Z) reacts with excess of at high temperature gives inorganic graphite

The major products obtained in the reaction of oxalic acid with conc. upon heating are
1.
2.
3.
4.
reacts with
1. Only water
2. Only acids
3. Only alkalis
4. Both acids and alkalis
All the products formed in the oxidation of , are
1.
2.
3.
4.
In the structure of borax, the numbers of boron atoms and B-O-B units, respectively, are
1. 4 and 5
2. 4 and 3
3. 5 and 4
4. 5 and 3
Hydrolysis of gives X which on treatment with sodium carbonate produces Y. X and Y, respectively, are
1.
2.
3.
4.
The element that combines with oxygen to give an amphoteric oxide is
1. N
2. P
3. Al
4. Na
What is the correct order of Lewis acid strength for the following compounds:\(B B r_3, B C l_3 \text {, and } B F_3\)?
1. < <
2. < <
3. < <
4. < <
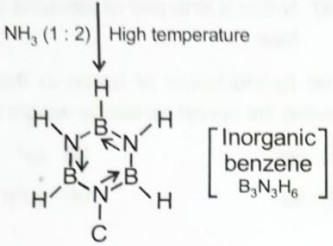
The product formed by the reaction of diborane and ammonia in the presence of heat is:
1.
2.
3.
4.
The bond energy of B-F bond in is 646 kJ , while that of N-F bond in is 280 kJ . This is because
1. N is more electronegative than B
2. The atomic mass of N is higher than that of B
3. The B-F bond gets a partial double bond character due to p-p overlap
4. N has a lone pair of electrons while B does not have






