The bond order for a species with the configuration will be
1. 1
2.
3. Zero
4. 3/2
Subtopic: M.O.T |
84%
Level 1: 80%+
Hints
Which molecule is least likely to form hydrogen bonds?
| 1. | \(\mathrm{NH}_3\) | 2. | \(\mathrm{H_2NOH}\) |
| 3. | \(\mathrm{HF}\) | 4. | \(\mathrm{CH_3F}\) |
Subtopic: van der Waal Force & Hydrogen Bonding |
64%
Level 2: 60%+
Hints
Which of the following compounds has zero dipole moment?
1.
2.
3.
4.
Subtopic: Polarity |
79%
Level 2: 60%+
Hints
The linear molecule among the following is:
1.
2.
3.
4.
Subtopic: V.S.E.P.R & V.B.T |
93%
Level 1: 80%+
Hints
The molecule having the highest dipole moment is
1.
2.
3.
4.
Subtopic: Polarity |
83%
Level 1: 80%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
The species which has triangular planar geometry is
1.
2.
3.
4.
Subtopic: Hybridisation |
71%
Level 2: 60%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
The correct sequence of stability for the given molecular species (from the given options) is:
1.
2.
3.
4.
Subtopic: M.O.T |
71%
Level 2: 60%+
Hints
Unlock IMPORTANT QUESTION
This question was bookmarked by 5 NEET 2025 toppers during their NEETprep journey. Get Target Batch to see this question.
✨ Perfect for quick revision & accuracy boost
Buy Target Batch
Access all premium questions instantly
From the given structures, the correct structure(s) of is are
l. 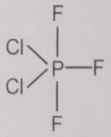
ll. 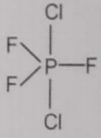
lll. 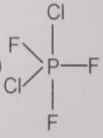
1. Only l
2. Only ll
3. Only lll
4. l, ll and lll
Subtopic: Hybridisation |
62%
Level 2: 60%+
Hints
The pair that is isostructural (i.e. having the same shape and hybridization) is
1.
2.
3.
4.
Subtopic: Hybridisation |
88%
Level 1: 80%+
Hints






