The figure below shows two paths that may be taken by a gas to go from state A to state C. In process AB, \(400~\text{J}\) of heat is added to the system and in process BC, \(100~\text{J}\) of heat is added to the system. The heat absorbed by the system in the process AC will be:
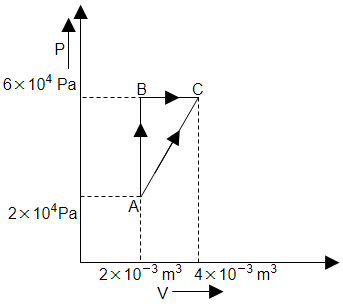
1.
\(380~\text{J}\)
2.
\(500~\text{J}\)
3.
\(460~\text{J}\)
4.
\(300~\text{J}\)

The coefficient of performance of a refrigerator is 5. If the temperature inside freezer is -20°C, the temperature of the surroundings to which it rejects heat is -
(1)31°C
(2)41°C
(3)11°C
(4)21°C
One mole of an ideal gas from an initial state A undergoes via two processes. It first undergoes isothermal expansion from volume V to 3V and then its volume is reduced from 3V to V at constant pressure. The correct p-V diagram representing the two processes is -
An ideal gas goes from state A to state B
via three different processes as indicated
in the p-V diagram
If indicates the heat absorbed
by the gas along the three processes and
indicates the change in
internal energy along the three processes
respectively, then
(a)
(b)
(c)
(d)
A container of volume 1m3 is divided into two equal compartments by a partition. One of these compartments contains an ideal gas at 300 K. The other compartment is vaccum. The whole system is thermally isolated from its surroundings. The partition is removed and the gas expands to occupy the whole volume of the container. Its temperature now would be -
(1) 300 K
(2) 239 K
(3) 200 K
(4) 100 K
A thermo-dynamical system is changed from state (P1, V1) to (P2, V2) by two different process. The quantity which will remain same will be
(1) ΔQ
(2) ΔW
(3) ΔQ + ΔW
(4) ΔQ – ΔW
If 150 J of heat is added to a system and the work done by the system is 110 J, then change in internal energy will be
(1) 260 J
(2) 150 J
(3) 110 J
(4) 40 J
If ΔQ and ΔW represent the heat supplied to the system and
the work done on the system, respectively, then the first law of thermodynamics can be written as: (where ΔU is the internal energy)
1. ΔQ = ΔU + ΔW
2. ΔQ = ΔU – ΔW
3. ΔQ = ΔW – ΔU
4. ΔQ = –ΔU – ΔW
In a thermodynamic process, pressure of a fixed mass of a gas is changed in such a manner that the gas molecules absorb 30 J of heat and 10 J of work is done by the gas. If the initial internal energy of the gas was 40 J, then the final internal energy will be -
(1) 30 J
(2) 20 J
(3) 60 J
(4) 40 J
Can two isothermal curves cut each other?
| 1. | Never |
| 2. | Yes |
| 3. | They will cut when the temperature is 0°C. |
| 4. | Yes, when the pressure is equal to the critical pressure. |








