\(1~\text g\) of water of volume \(1~\text{cm}^3\) at \(100^\circ \text{C}\) is converted into steam at the same temperature under normal atmospheric pressure \(\approx 1\times10^{5}~\text{Pa}.\) The volume of steam formed equals \(1671~\text{cm}^3.\) If the specific latent heat of vaporization of water is \(2256~\text{J/g},\) the change in internal energy is:
1. \(2423~\text J\)
2. \(2089~\text J\)
3. \(167~\text J\)
4. \(2256~\text J\)
1. \(2423~\text J\)
2. \(2089~\text J\)
3. \(167~\text J\)
4. \(2256~\text J\)
Which of the following thermodynamic quantities is an outcome of the second law of thermodynamics ?
1. Work
2. Enthalpy
3. Internal energy
4. Entropy
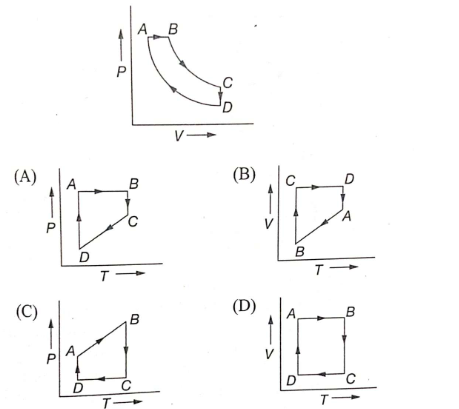
A cyclic process ABCD is shown in the P-V diagram. Which of the following curves represent the same process?
An ideal gas undergoes a cyclic process ABCA as shown. The heat exchange between the system and the surrounding during the process will be:
| 1. | 10 J | 2. | 5 J |
| 3. | 15 J | 4. | 20 J |
The molar heat capacity for an ideal gas
1. cannot be negative
2. must be equal to either or
3. must lie in the range
4. may have any value between and
An ideal gas expands according to the law = const. The molar heat capacity C is :
1.
2.
3.
4.
The molar heat capacity C for an ideal gas going through a given process is given by C = a/T , where 'a' is a constant. If , the work done by one mole of gas during heating from to through the given process will be:
1.
2.
3.
4. none of these

The pressure of a monoatomic gas increases linearly from N/m2 to N/m2 when its volume increases from 0.2 m3 to 0.5 m3. The molar heat capacity of the gas is:
[R = 8.31 J/mol k]
1. 20.1 J/molK
2. 17.14 J/molK
3. 18.14 J/molK
4. 20.14 J/molK
What is the nature of change in internal energy in the following three thermodynamic processes shown in figure?
(1) is positive in all the three cases
(2) is negative in all the three cases
(3) is positive for (i), negative for (ii), zero for (iii)
(4) = 0, in all the cases








