n moles of an ideal gas is heated at constant pressure
from 50°C to 100°C, the increase in internal energy
of the gas is
1.
2.
3.
4.
from 50°C to 100°C, the increase in internal energy
of the gas is
A and B are two adiabatic curves for two different gases.
Then A and B corresponds to :
1. Ar and He respectively.
2. He and H respectively.
3. O and H respectively.
4. H and He respectively
An ideal gas going through the reversible cycle , has the V-T diagram as shown below in the figure. Process are adiabatic.
The corresponding P-V diagram for the process is (all figures are schematic and not drawn to scale):
1.
2.
3.
4.
What is the nature of change in internal energy in the following three thermodynamic processes shown in figure?
(1) is positive in all the three cases
(2) is negative in all the three cases
(3) is positive for (i), negative for (ii), zero for (iii)
(4) = 0, in all the cases
An ideal gas undergoes a cyclic process ABCA as shown. The heat exchange between the system and the surrounding during the process will be:
| 1. | 10 J | 2. | 5 J |
| 3. | 15 J | 4. | 20 J |
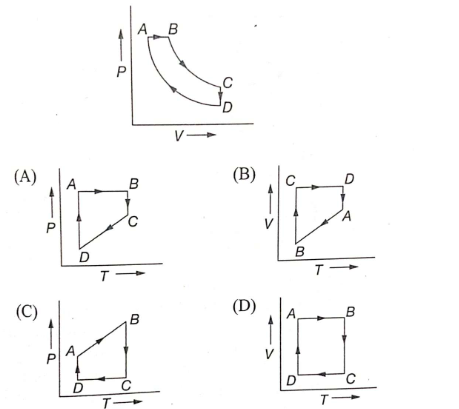
A cyclic process ABCD is shown in the P-V diagram. Which of the following curves represent the same process?
A gas mixture consists of 2 moles of and 4 moles of Ar at temperature T. Neglecting all vibrational modes, the total internal energy of the system is
1. 4 RT
2. 15 RT
3. 9 RT
4. 11 RT
\(1~\text g\) of water of volume \(1~\text{cm}^3\) at \(100^\circ \text{C}\) is converted into steam at the same temperature under normal atmospheric pressure \(\approx 1\times10^{5}~\text{Pa}.\) The volume of steam formed equals \(1671~\text{cm}^3.\) If the specific latent heat of vaporization of water is \(2256~\text{J/g},\) the change in internal energy is:
| 1. | \(2423~\text J\) | 2. | \(2089~\text J\) |
| 3. | \(167~\text J\) | 4. | \(2256~\text J\) |
Equal moles of monoatomic and diatomic gases are
mixed, for mixture is
1. 1.5
2. 1
3. 2
4. 1.67
Which of the following thermodynamic quantities is an outcome of the second law of thermodynamics ?
1. Work
2. Enthalpy
3. Internal energy
4. Entropy














