1. Natural rubber
Rubber is a natural polymer and possesses elastic properties. It is also termed as elastomer and has a variety of uses. It is manufactured from rubber latex which is a colloidal dispersion of rubber in water. This latex is obtained from the bark of rubber tree and is found in India, Srilanka, Indonesia, Malaysia and South America.
Natural rubber may be considered as a linear polymer of isoprene (2-methyl-1, 3-butadiene) and is also called as cis - 1, 4 - polyisoprene.
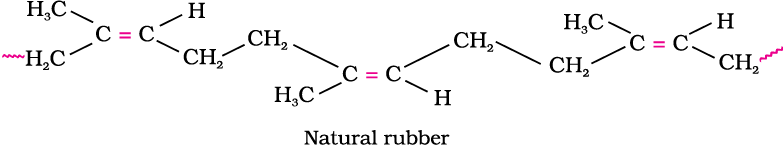
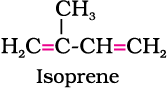
The cis-polyisoprene molecule consists of various chains held together by weak van der Waals interactions and has a coiled structure. Thus, it can be stretched like a spring and exhibits elastic properties.
Vulcanisation of rubber: Natural rubber becomes soft at high temperature (>335 K) and brittle at low temperatures (<283 K) and shows high water absorption capacity. It is soluble in non-polar solvents and is non-resistant to attack by oxidising agents. To improve upon these physical properties, a process of vulcanisation is carried out. This process consists of heating a mixture of raw rubber with sulphur and an appropriate additive at a temperature range between 373 K to 415 K. On vulcanisation, sulphur forms cross links at the reactive sites of double bonds and thus the rubber gets stiffened.
In the manufacture of tyre rubber, 5% of sulphur is used as a crosslinking agent. The probable structures of vulcanised rubber molecules are depicted below:
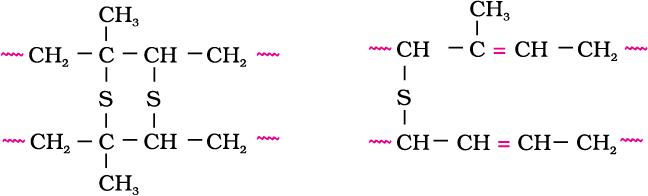
2. Synthetic rubbers
Synthetic rubber is any vulcanisable rubber like polymer, which is capable of getting stretched to twice its length. However, it returns to its original shape and size as soon as the external stretching force is released. Thus, synthetic rubbers are either homopolymers of
1, 3 - butadiene derivatives or copolymers of 1, 3 - butadiene or its derivatives with another unsaturated monomer.
Preparation of Synthetic Rubbers
1. Neoprene
Neoprene or polychloroprene is formed by the free radical polymerisation of chloroprene.

It has superior resistance to vegetable and mineral oils. It is used for manufacturing conveyor belts, gaskets and hoses.
2. Buna – N
You have already studied about Buna-S, in Section 15.1.3. Buna –N is obtained by the copolymerisation of 1, 3 – butadiene and acrylonitrile in the presence of a peroxide catalyst.

It is resistant to the action of petrol, lubricating oil and organic solvents. It is used in making oil seals, tank lining, etc.
Intext Questions
15.5 Explain the difference between Buna-N and Buna-S.
15.6 Arrange the following polymers in increasing order of their intermolecular forces.
(i) Nylon 6,6, Buna-S, Polythene.
(ii) Nylon 6, Neoprene, Polyvinyl chloride.