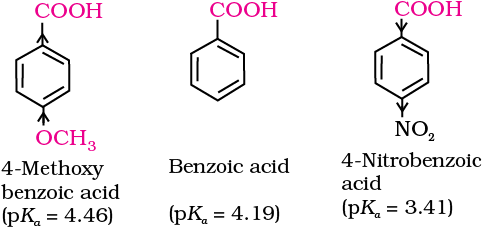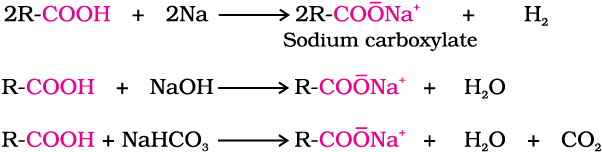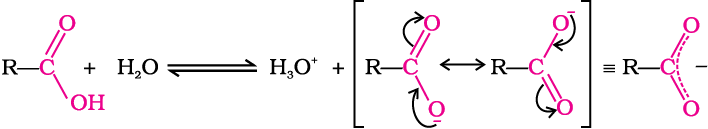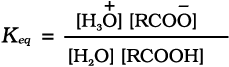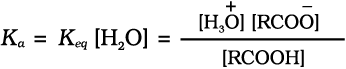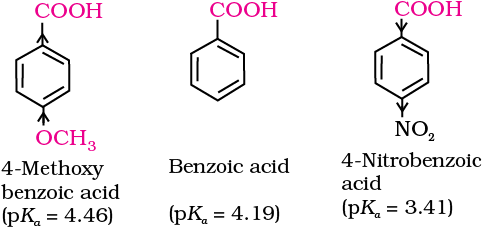12.9.1 Reactions Involving Cleavage of O–H Bond
Acidity
Reactions with metals and alkalies
The carboxylic acids like alcohols evolve hydrogen with electropositive metals and form salts with alkalies similar to phenols. However, unlike phenols they react with weaker bases such as carbonates and hydrogencarbonates to evolve carbon dioxide. This reaction is used to detect the presence of carboxyl group in an organic compound.
Carboxylic acids dissociate in water to give resonance stabilised carboxylate anions and hydronium ion.
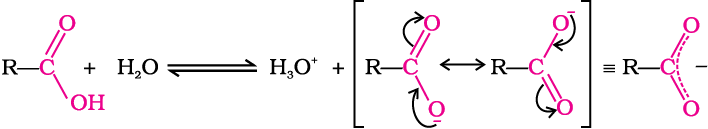
where Keq, is equilibrium constant and Ka is the acid dissociation constant.
For convenience, the strength of an acid is generally indicated by its pka value rather than its Ka value.
pKa = – log Ka
The pKa of hydrochloric acid is –7.0, where as pKa of trifluoroacetic acid (the strongest carboxylic acid), benzoic acid and acetic acid are 0.23, 4.19 and 4.76, respectively.
Smaller the pKa, the stronger the acid ( the better it is as a proton donor). Strong acids have pKa values < 1, the acids with pKa values between 1 and 5 are considered to be moderately strong acids, weak acids have pKa values between 5 and 15, and extremely weak acids have pKa values >15.
Carboxylic acids are weaker than mineral acids, but they are stronger acids than alcohols and many simple phenols (pKa is ~16 for ethanol and 10 for phenol). In fact, carboxylic acids are amongst the most acidic organic compounds you have studied so far. You already know why phenols are more acidic than alcohols. The higher acidity of carboxylic acids as compared to phenols can be understood similarly. The conjugate base of carboxylic acid, a carboxylate ion, is stabilised by two equivalent resonance structures in which the negative charge is at the more electronegative oxygen atom. The conjugate base of phenol, a phenoxide ion, has non-equivalent resonance structures in which the negative charge is at the less electronegative carbon atom. Therefore, resonance in phenoxide ion is not as important as it is in carboxylate ion. Further, the negative charge is delocalised over two electronegative oxygen atoms in carboxylate ion whereas it is less effectively delocalised over one oxygen atom and less electronegative carbon atoms in phenoxide ion (Unit 11, Class XII). Thus, the carboxylate ion is more stabilised than phenoxide ion, so carboxylic acids are more acidic than phenols.
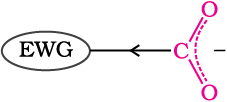
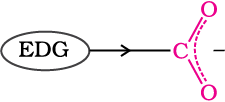
Effect of substituents on the acidity of carboxylic acids: Substituents may affect the stability of the conjugate base and thus, also affect the acidity of the carboxylic acids. Electron withdrawing groups increase the acidity of carboxylic acids by stabilising the conjugate base through delocalisation of the negative charge by inductive and/or resonance effects. Conversely, electron donating groups decrease the acidity by destabilising the conjugate base.
Electron withdrawing group (EWG) stabilises the carboxylate anion and strengthens the acid
Electron donating group (EDG) destabilises the carboxylate anion and weakens the acid
The effect of the following groups in increasing acidity order is
Ph < I < Br < Cl < F < CN < NO2 < CF3
Thus, the following acids are arranged in order of increasing acidity (based on pKa values):
CF3COOH > CCl3COOH > CHCl2COOH > NO2CH2COOH > NC-CH2COOH >

FCH2COOH > ClCH2COOH > BrCH2COOH > HCOOH > ClCH2CH2COOH >
(continue)
C6H5COOH > C6H5CH2COOH > CH3COOH > CH3CH2COOH
(continue )
Direct attachment of groups such as phenyl or vinyl to the carboxylic acid, increases the acidity of corresponding carboxylic acid, contrary to the decrease expected due to resonance effect shown below:
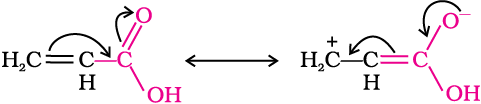
This is because of greater electronegativity of sp2 hybridised carbon to which carboxyl carbon is attached. The presence of electron withdrawing group on the phenyl of aromatic carboxylic acid increases their acidity while electron donating groups decrease their acidity.