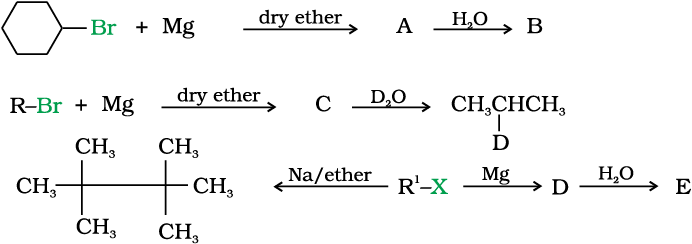1. Nucleophilic substitution
Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
(i) Resonance effect : In haloarenes, the electron pairs on halogen atom are in conjugation with π-electrons of the ring and the following resonating structures are possible.
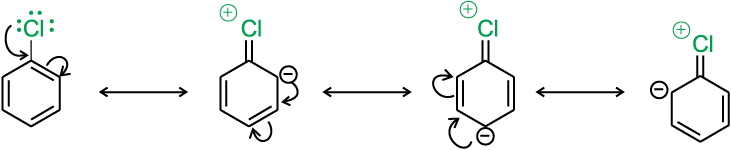
C—Cl bond acquires a partial double bond character due to resonance. As a result, the bond cleavage in haloarene is difficult than haloalkane and therefore, they are less reactive towards nucleophilic substitution reaction.
(ii) Difference in hybridisation of carbon atom in C—X bond: In haloalkane, the carbon atom attached to halogen is sp3 hybridised while in case of haloarene, the carbon atom attached to halogen is sp2-hybridised.
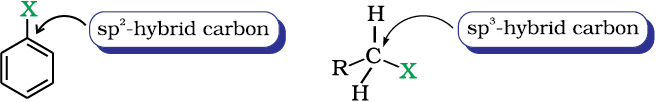
The sp2 hybridised carbon with a greater s-character is more electronegative and can hold the electron pair of C—X bond more tightly than sp3-hybridised carbon in haloalkane with less s-chararcter. Thus, C—Cl bond length in haloalkane is 177pm while in haloarene is 169 pm. Since it is difficult to break a shorter bond than a longer bond, therefore, haloarenes are less reactive than haloalkanes towards nucleophilic substitution reaction.
(iii) Instability of phenyl cation: In case of haloarenes, the phenyl cation formed as a result of self-ionisation will not be stabilised by resonance and therefore, SN1 mechanism is ruled out.
(iv) Because of the possible repulsion, it is less likely for the electron rich nucleophile to approach electron rich arenes.
Replacement by hydroxyl group
Chlorobenzene can be converted into phenol by heating in aqueous sodium hydroxide solution at a temperature of 623K and a pressure of 300 atmospheres.
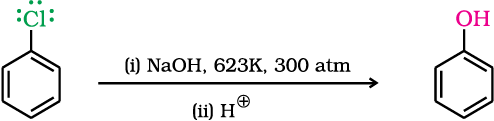
The presence of an electron withdrawing group (-NO2) at ortho- and para-positions increases the reactivity of haloarenes.
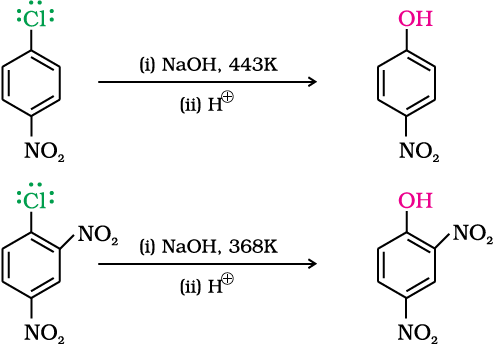
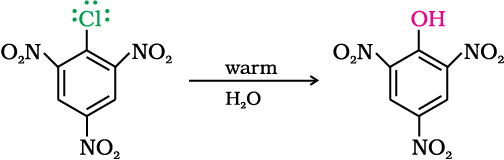
The effect is pronounced when (-NO2) group is introduced at ortho- and para- positions. However, no effect on reactivity of haloarenes is observed by the presence of electron withdrawing group at meta-position. Mechanism of the reaction is as depicted:
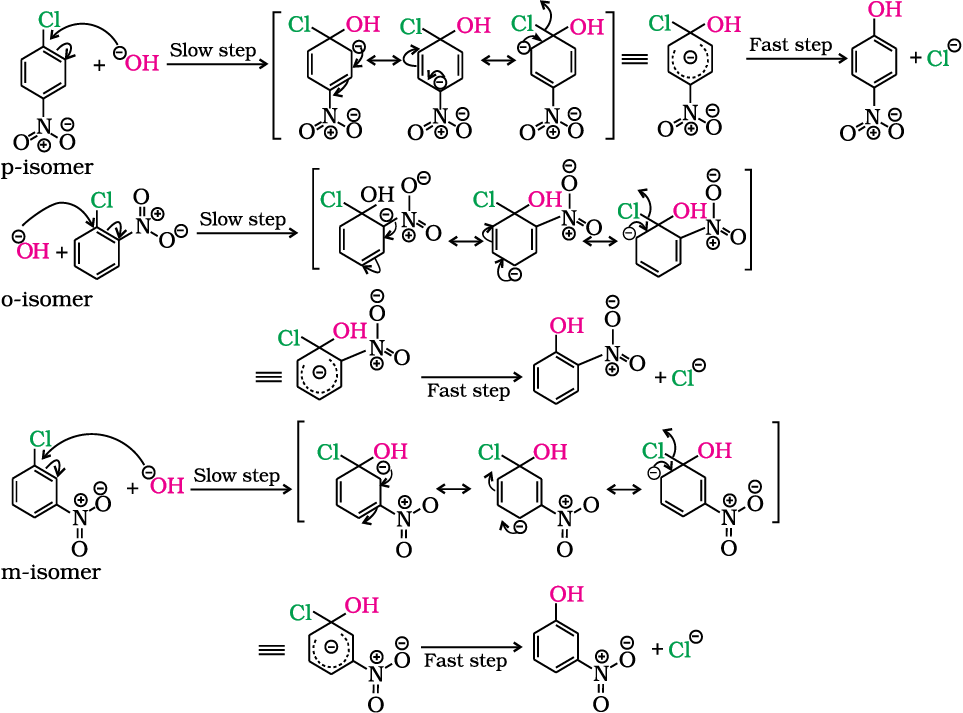
Can you think why does NO2 group show its effect only at ortho- and para- positions and not at meta- position?
As shown, the presence of nitro group at ortho- and para-positions withdraws the electron density from the benzene ring and thus facilitates the attack of the nucleophile on haloarene. The carbanion thus formed is stabilised through resonance. The negative charge appeared at ortho- and para- positions with respect to the halogen substituent is stabilised by –NO2 group while in case of meta-nitrobenzene, none of the resonating structures bear the negative charge on carbon atom bearing the –NO2 group. Therefore, the presence of nitro group at meta- position does not stabilise the negative charge and no effect on reactivity is observed by the presence of –NO2 group at meta-position.
2. Electrophilic substitution reactions
Haloarenes undergo the usual electrophilic reactions of the benzene ring such as halogenation, nitration, sulphonation and Friedel-Crafts reactions. Halogen atom besides being slightly deactivating is o, p-directing; therefore, further substitution occurs at ortho- and para-positions with respect to the halogen atom. The o, p-directing influence of halogen atom can be easily understood if we consider the resonating structures of halobenzene as shown:
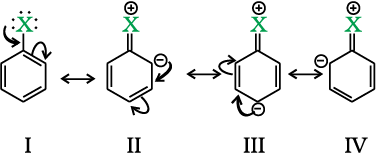
Due to resonance, the electron density increases more at ortho- and para-positions than at meta-positions. Further, the halogen atom because of its –I effect has some tendency to withdraw electrons from the benzene ring. As a result, the ring gets somewhat deactivated as compared to benzene and hence the electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions as compared to those in benzene.
(i) Halogenation
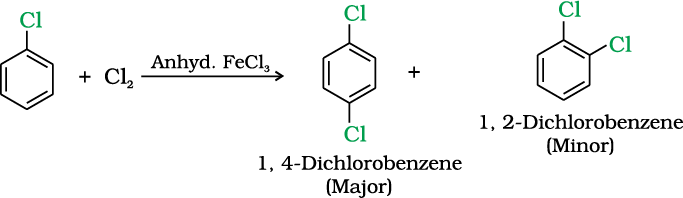
(ii) Nitration
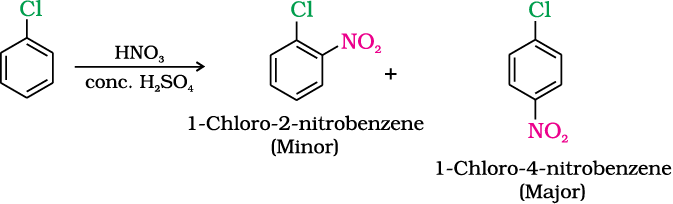
(iii) Sulphonation
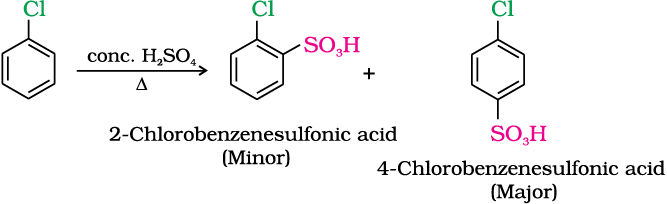
(iv) Friedel-Crafts reaction
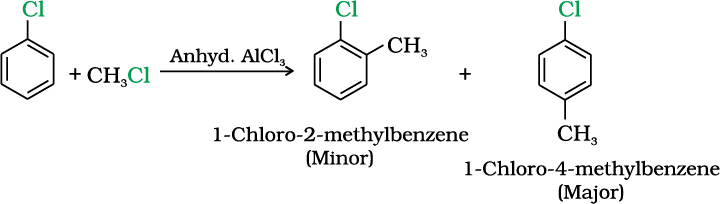
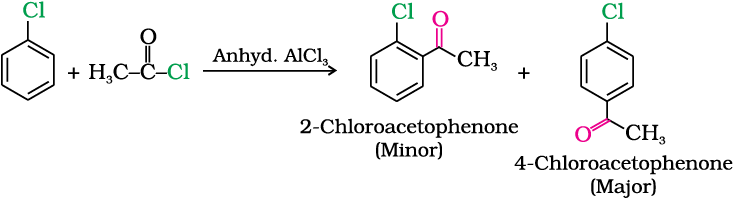
Example 10.9
Although chlorine is an electron withdrawing group, yet it is ortho-, para- directing in electrophilic aromatic substitution reactions. Why?
Solution
Chlorine withdraws electrons through inductive effect and releases electrons through resonance. Through inductive effect, chlorine destabilises the intermediate carbocation formed during the electrophilic substitution.
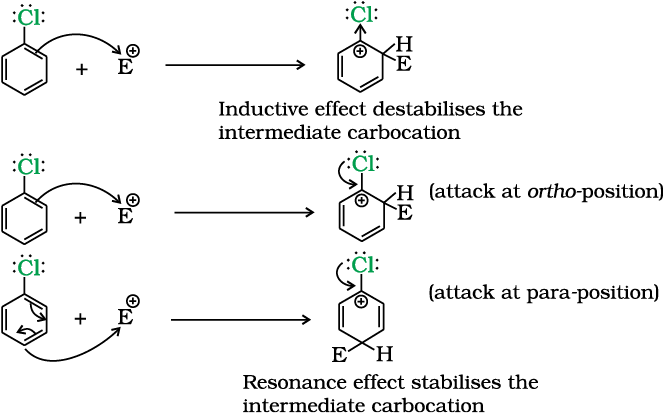
Through resonance, halogen tends to stabilise the carbocation and the effect is more pronounced at ortho- and para- positions. The inductive effect is stronger than resonance and causes net electron withdrawal and thus causes net deactivation. The resonance effect tends to oppose the inductive effect for the attack at ortho- and para- positions and hence makes the deactivation less for ortho- and para- attack. Reactivity is thus controlled by the stronger inductive effect and orientation is controlled by resonance effect.
3. Reaction with metals
Wurtz-Fittig reaction
A mixture of an alkyl halide and aryl halide gives an alkylarene when treated with sodium in dry ether and is called Wurtz-Fittig reaction.

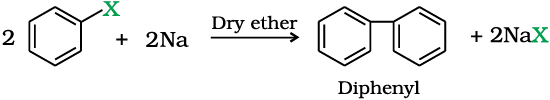
Fittig reaction
Aryl halides also give analogous compounds when treated with sodium in dry ether, in which two aryl groups are joined together. It is called Fittig reaction.
Intext Questions
10.7 Which alkyl halide from the following pairs would you expect to react more rapidly by an SN2 mechanism? Explain your answer.
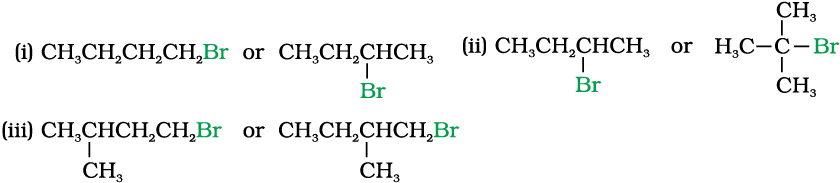
10.8 In the following pairs of halogen compounds, which compound undergoes faster SN1 reaction?
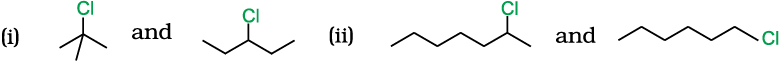
10.9 Identify A, B, C, D, E, R and R1 in the following: