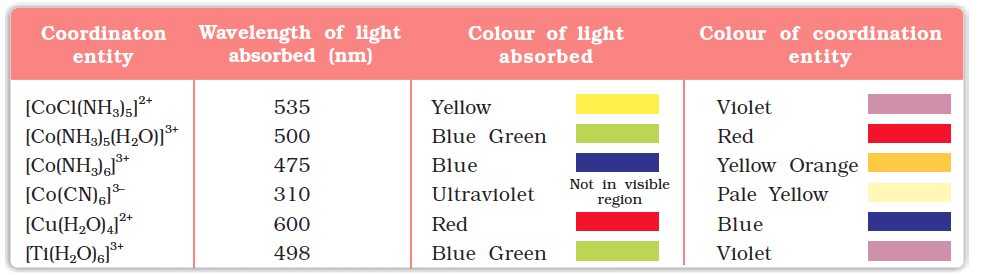
In the previous Unit, we learnt that one of the most distinctive properties of transition metal complexes is their wide range of colours. This means that some of the visible spectrum is being removed from white light as it passes through the sample, so the light that emerges is no longer white. The colour of the complex is complementary to that which is absorbed. The complementary colour is the colour generated from the wavelength left over; if green light is absorbed by the complex, it appears red. Table 9.3 gives the relationship of the different wavelength absorbed and the colour observed.
Table 9.3: Relationship between the Wavelength of Light absorbed and the Colour observed in some Coordination Entities
The colour in the coordination compounds can be readily explained in terms of the crystal field theory. Consider, for example, the complex [Ti(H2O)6]3+, which is violet in colour. This is an octahedral complex where the single electron (Ti3+ is a 3d1system) in the metal d orbital is in the t2g level in the ground state of the complex. The next higher state available for the electron is the empty eg level. If light corresponding to the energy of blue-green region is absorbed by the complex, it would excite the electron from t2g level to the eg level (t2g1eg0 → t2g0eg1). Consequently, the complex appears violet in colour (Fig. 9.10). The crystal field theory attributes the colour of the coordination compounds to d-d transition of the electron.
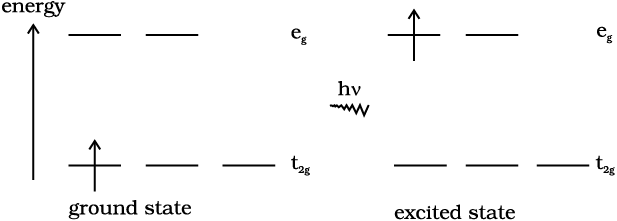
Fig.9.10: Transition of an electron in [Ti(H2O)6]3+
[Ni(H2O)6]2+ (aq) + en (aq) = [Ni(H2O)4(en)]2+(aq) + 2H2O
green pale blue
[Ni(H2O)4 (en)]2+(aq) + en (aq) = [Ni(H2O)2(en)2]2+(aq) + 2H2O
blue/purple
[Ni(H2O)2(en)2]2+(aq) + en (aq) = [Ni(en)3]2+(aq) + 2H2O
violet
This sequence is shown in Fig. 9.11.
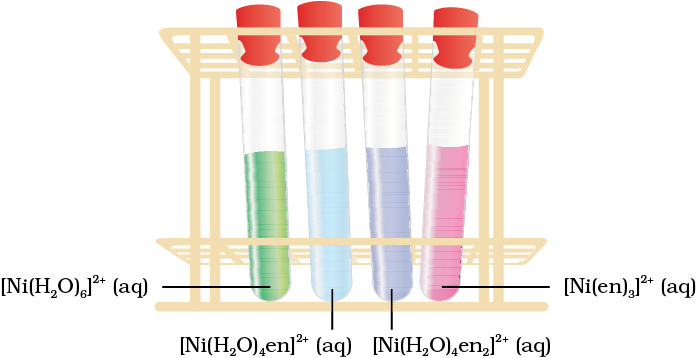
Fig. 9.11 Aqueous solutions of complexes of nickel(II) with an increasing number of ethane-1, 2-diamine ligands.
Colour of Some Gem Stones
The colours produced by electronic transitions within the d orbitals of a transition metal ion occur frequently in everyday life. Ruby [Fig.9.12(a)] is aluminium oxide (Al2O3) containing about 0.5-1% Cr3+ ions (d3), which are randomly distributed in positions normally occupied by Al3+. We may view these chromium(III) species as octahedral chromium(III) complexes incorporated into the alumina lattice; d–dtransitions at these centres give rise to the colour.
In emerald [Fig.9.12(b)], Cr3+ ions occupy octahedral sites in the mineral beryl (Be3Al2Si6O18). The absorption bands seen in the ruby shift to longer wavelength, namely yellow-red and blue, causing emerald to transmit light in the green region.
(a) (b)
(b) 
Fig.9.12: (a) Ruby: this gemstone was found in marble from Mogok, Myanmar; (b) Emerald: this gemstone was found in Muzo, Columbia.