The metals of the first series with the exception of copper are relatively more reactive and are oxidised by 1M H+, though the actual rate at which these metals react with oxidising agents like hydrogen ion (H+) is sometimes slow. For example, titanium and vanadium, in practice, are passive to dilute non oxidising acids at room temperature. The EƟ values for M2+/M (Table 8.2) indicate a decreasing tendency to form divalent cations across the series. This general trend towards less negative EƟ values is related to the increase in the sum of the first and second ionisation enthalpies. It is interesting to note that the EƟ values for Mn, Ni and Zn are more negative than expected from the general trend. Whereas the stabilities of half-filled d subshell (d5) in Mn2+ and completely filled d subshell (d10) in zinc are related to their E e values; for nickel, EƟ value is related to the highest negative enthalpy of hydration.
An examination of the EƟ values for the redox couple M3+/M2+ (Table 8.2) shows that Mn3+ and Co3+ ions are the strongest oxidising agents in aqueous solutions. The ions Ti2+, V2+ and Cr2+ are strong reducing agents and will liberate hydrogen from a dilute acid, e.g.,
2 Cr2+(aq) + 2 H+(aq) → 2 Cr3+(aq) + H2(g)
Example 8.6
For the first row transition metals the EƟ values are:
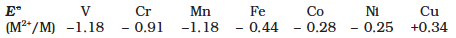
Explain the irregularity in the above values.
Solution
The EƟ (M2+/M) values are not regular which can be explained from the irregular variation of ionisation enthalpies
(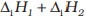
Example 8.7
Why is the EƟ value for the Mn3+/Mn2+ couple much more positive than that for Cr3+/Cr2+ or Fe3+/Fe2+? Explain.
Solution
Much larger third ionisation energy of Mn (where the required change is d5 to d4) is mainly responsible for this. This also explains why the +3 state of Mn is of little importance.
8.6 Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
8.7 Which is a stronger reducing agent Cr2+ or Fe2+ and why ?