An examination of the EƟ(M3+/M2+) values (Table 8.2) shows the varying trends. The low value for Sc reflects the stability of Sc3+ which has a noble gas configuration. The highest value for Zn is due to the removal of an electron from the stable d10 configuration of Zn2+. The comparatively high value for Mn shows that Mn2+(d5) is particularly stable, whereas comparatively low value for Fe shows the extra stability of Fe3+ (d5). The comparatively low value for V is related to the stability of V2+ (half-filled t2g level, Unit 9).
Table 8.5 shows the stable halides of the 3d series of transition metals. The highest oxidation numbers are achieved in TiX4 (tetrahalides), VF5 and CrF6. The +7 state for Mn is not represented in simple halides but MnO3F is known, and beyond Mn no metal has a trihalide except FeX3 and CoF3. The ability of fluorine to stabilise the highest oxidation state is due to either higher lattice energy as in the case of CoF3, or higher bond enthalpy terms for the higher covalent compounds, e.g., VF5 and CrF6.
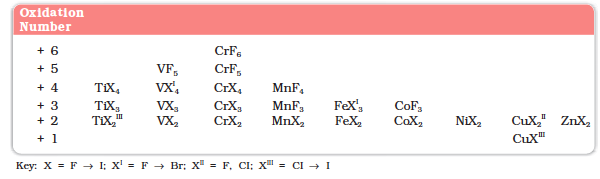
Although V+5 is represented only by VF5, the other halides, however, undergo hydrolysis to give oxohalides, VOX3. Another feature of fluorides is their instability in the low oxidation states e.g., VX2 (X = CI, Br or I) and the same applies to CuX. On the other hand, all CuII halides are known except the iodide. In this case, Cu2+ oxidises I–to I2:
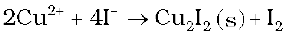
However, many copper (I) compounds are unstable in aqueous solution and undergo disproportionation.
2Cu+ → Cu2+ + Cu
The stability of Cu2+ (aq) rather than Cu+(aq) is due to the much more negative ∆hydHƟ of Cu2+ (aq) than Cu+, which more than compensates for the second ionisation enthalpy of Cu.
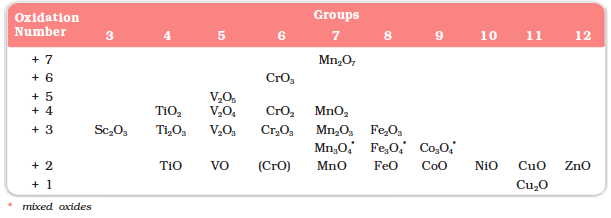
The ability of oxygen to stabilise the highest oxidation state is demonstrated in the oxides. The highest oxidation number in the oxides (Table 8.6) coincides with the group number and is attained in Sc2O3 to Mn2O7. Beyond Group 7, no higher oxides of Fe above Fe2O3, are known, although ferrates (VI)(FeO4)2–, are formed in alkaline media but they readily decompose to Fe2O3 and O2. Besides the oxides, oxocations stabilise Vv as VO2+, VIV as VO2+ and TiIV as TiO2+. The ability of oxygen to stabilise these high oxidation states exceeds that of fluorine. Thus the highest Mn fluoride is MnF4 whereas the highest oxide is Mn2O7. The ability of oxygen to form multiple bonds to metals explains its superiority. In the covalent oxide Mn2O7, each Mn is tetrahedrally surrounded by O’s including a Mn–O–Mn bridge. The tetrahedral [MO4]n- ions are known for VV, CrVl, MnV, MnVl and MnVII.
Example 8.5
How would you account for the increasing oxidising power in the series VO2+ < Cr2O72– < MnO4 – ?
Solution
This is due to the increasing stability of the lower species to which they are reduced.
Intext Question
8.5 How would you account for the irregular variation of ionisation enthalpies (first and second) in the first series of the transition elements?