Some of the important characteristics of both types of adsorption are described below:
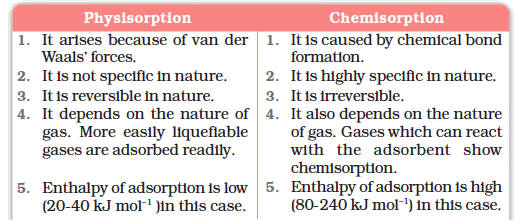
Characteristics of physisorption
(i) Lack of specificity: A given surface of an adsorbent does not show any preference for a particular gas as the van der Waals’ forces are universal.
(ii) Nature of adsorbate: The amount of gas adsorbed by a solid depends on the nature of gas. In general, easily liquefiable gases (i.e., with higher critical temperatures) are readily adsorbed as van der Waals’ forces are stronger near the critical temperatures. Thus, 1g of activated charcoal adsorbs more sulphur dioxide (critical temperature 630K), than methane (critical temperature 190K) which is still more than 4.5 mL of dihydrogen (critical temperature 33K).
(iii) Reversible nature: Physical adsorption of a gas by a solid is generally reversible. Thus,
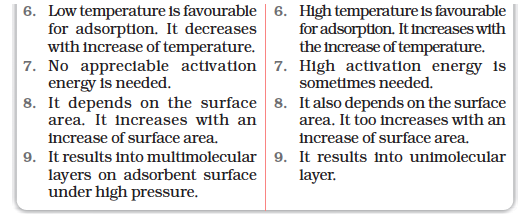
(iv) Surface area of adsorbent: The extent of adsorption increases with the increase of surface area of the adsorbent. Thus, finely divided metals and porous substances having large surface areas are good adsorbents.
(v) Enthalpy of adsorption: No doubt, physical adsorption is an exothermic process but its enthalpy of adsorption is quite low (20–40 kJ mol-1). This is because the attraction between gas molecules and solid surface is only due to weak van der Waals’ forces.
Characteristics of chemisorption
(i) High specificity: Chemisorption is highly specific and it will only occur if there is some possibility of chemical bonding between adsorbent and adsorbate. For example, oxygen is adsorbed on metals by virtue of oxide formation and hydrogen is adsorbed by transition metals due to hydride formation.
(ii) Irreversibility: As chemisorption involves compound formation, it is usually irreversible in nature. Chemisorption is also an exothermic process but the process is very slow at low temperatures on account of high energy of activation. Like most chemical changes, adsorption often increases with rise of temperature. Physisorption of a gas adsorbed at low temperature may change into chemisorption at a high temperature. Usually high pressure is also favourable for chemisorption.
(iii) Surface area: Like physical adsorption, chemisorption also increases with increase of surface area of the adsorbent.