Matter normally exists in three states: solid, liquid and gas. A transition from one of these states to another is called a change of state. Two common changes of states are solid to liquid and liquid to gas (and, vice versa). These changes can occur when the exchange of heat takes place between the substance and its surroundings. To study the change of state on heating or cooling, let us perform the following activity.
Take some cubes of ice in a beaker. Note the temperature of ice. Start heating it slowly on a constant heat source. Note the temperature after every minute. Continuously stir the mixture of water and ice. Draw a graph between temperature and time (Fig. 11.9). You will observe no change in the temperature as long as there is ice in the beaker. In the above process, the temperature of the system does not change even though heat is being continuously supplied. The heat supplied is being utilised in changing the state from solid (ice) to liquid (water).
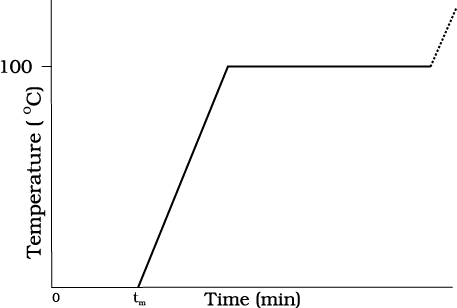
Fig. 11.9 A plot of temperature versus time showing the changes in the state of ice on heating (not to scale).
The change of state from solid to liquid is called melting and from liquid to solid is called fusion. It is observed that the temperature remains constant until the entire amount of the solid substance melts. That is, both the solid and the liquid states of the substance coexist in thermal equilibrium during the change of states from solid to liquid. The temperature at which the solid and the liquid states of the substance is in thermal equilibrium with each other is called its melting point. It is characteristic of the substance. It also depends on pressure. The melting point of a substance at standard atomspheric pressure is called its normal melting point. Let us do the following activity to understand the process of melting
of ice.
Take a slab of ice. Take a metallic wire and fix two blocks, say 5 kg each, at its ends. Put the wire over the slab as shown in Fig. 11.10. You will observe that the wire passes through the ice slab. This happens due to the fact that just below the wire, ice melts at lower temperature due to increase in pressure. When the wire has passed, water above the wire freezes again. Thus, the wire passes through the slab and the slab does not split. This phenomenon of refreezing is called regelation. Skating is possible on snow due to the formation of water under the skates. Water is formed due to the increase of pressure and it acts as a lubricant.
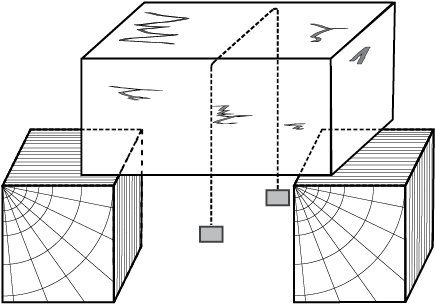
Fig. 11.10
After the whole of ice gets converted into water and as we continue further heating, we shall see that temperature begins to rise (Fig.11.9). The temperature keeps on rising till it reaches nearly 100 °C when it again becomes steady. The heat supplied is now being utilised to change water from liquid state to vapour or gaseous state.
The change of state from liquid to vapour (or gas) is called vaporisation. It is observed that the temperature remains constant until the entire amount of the liquid is converted into vapour. That is, both the liquid and vapour states of the substance coexist in thermal equilibrium, during the change of state from liquid to vapour. The temperature at which the liquid and the vapour states of the substance coexist is called its boiling point. Let us do the following activity to understand the process of boiling of water.
Take a round-bottom flask, more than half filled with water. Keep it over a burner and fix a thermometer and steam outlet through the cork of the flask (Fig. 11.11). As water gets heated in the flask, note first that the air, which was dissolved in the water, will come out as small bubbles. Later, bubbles of steam will form at the bottom but as they rise to the cooler water near the top, they condense and disappear. Finally, as the temperature of the entire mass of the water reaches 100 °C, bubbles of steam reach the surface and boiling is said to occur. The steam in the flask may not be visible but as it comes out of the flask, it condenses as tiny droplets of water, giving a foggy appearance.