In the presence of peroxide, addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov rule. This happens only with HBr but not with HCl and Hl. This addition reaction was observed by M.S. Kharash and F.R. Mayo in 1933 at the University of Chicago. This reaction is known as peroxide or Kharash effect or addition reaction anti to Markovnikov rule.
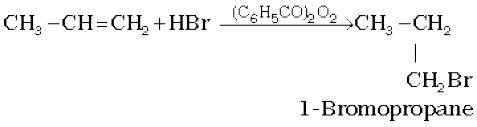 (13.43)
(13.43)
Mechanism : Peroxide effect proceeds via free radical chain mechanism as given below:
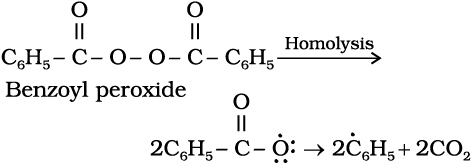
(ii) 
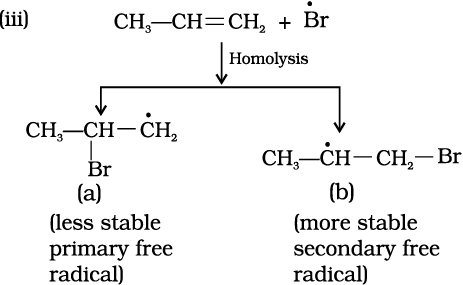
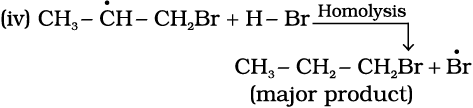
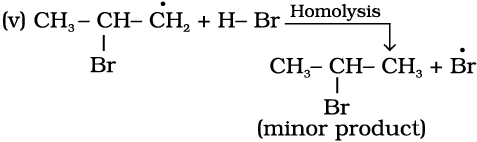
The secondary free radical obtained in the above mechanism (step iii) is more stable than the primary. This explains the formation of 1-bromopropane as the major product. It may be noted that the peroxide effect is not observed in addition of HCl and HI. This may be due to the fact that the H–Cl bond being stronger (430.5 kJ mol–1) than H–Br bond (363.7 kJ mol–1), is not cleaved by the free radical, whereas the H–I bond is weaker (296.8 kJ mol–1) and iodine free radicals combine to form iodine molecules instead of adding to the double bond.
Problem 13.12
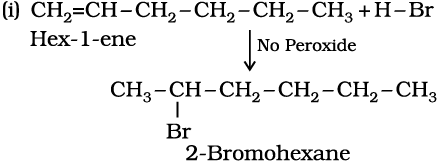
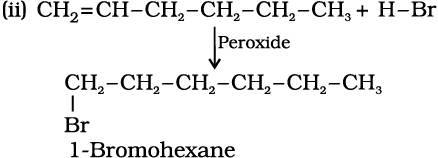
Write IUPAC names of the products obtained by addition reactions of HBr to hex-1-ene
(i) in the absence of peroxide and
(ii) in the presence of peroxide.
Solution
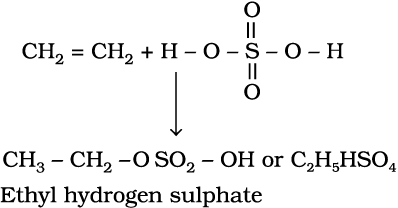
4. Addition of sulphuric acid : Cold concentrated sulphuric acid adds to alkenes in accordance with Markovnikov rule to form alkyl hydrogen sulphate by the electrophilic addition reaction.
 (13.44)
(13.44)
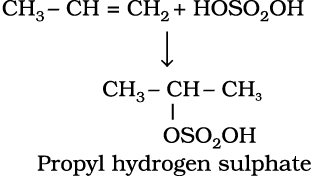 (13.45)
(13.45)
5. Addition of water : In the presence of a few drops of concentrated sulphuric acid alkenes react with water to form alcohols, in accordance with the Markovnikov rule.
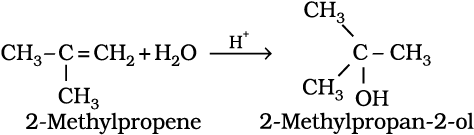 (13.46)
(13.46)
6. Oxidation: Alkenes on reaction with cold, dilute, aqueous solution of potassium permanganate (Baeyer’s reagent) produce vicinal glycols. Decolorisation of KMnO4 solution is used as a test for unsaturation.
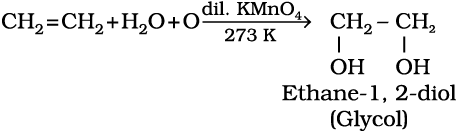 (13.47)
(13.47)
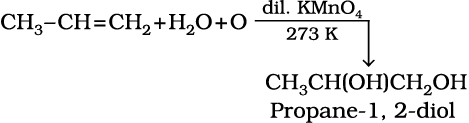 (13.48)
(13.48)
b) Acidic potassium permanganate or acidic potassium dichromate oxidises alkenes to ketones and/or acids depending upon the nature of the alkene and the experimental conditions
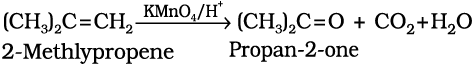 (13.49)
(13.49)
 (13.50)
(13.50)
7. Ozonolysis : Ozonolysis of alkenes involves the addition of ozone molecule to alkene to form ozonide, and then cleavage of the ozonide by Zn-H2O to smaller molecules. This reaction is highly useful in detecting the position of the double bond in alkenes or other unsaturated compounds.
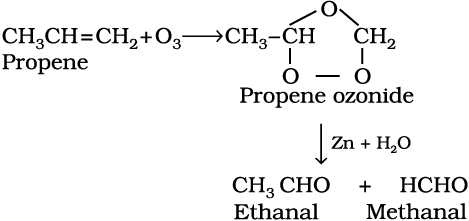 (13.51)
(13.51)
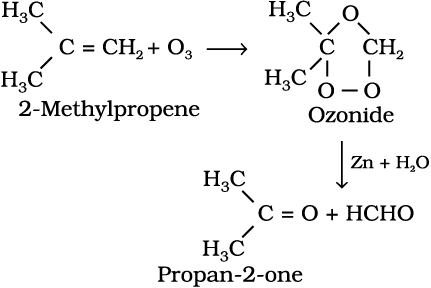 (13.52)
(13.52)
8. Polymerisation: You are familiar with polythene bags and polythene sheets. Polythene is obtained by the combination of large number of ethene molecules at high temperature, high pressure and in the presence of a catalyst. The large molecules thus obtained are called polymers. This reaction is known as polymerisation. The simple compounds from which polymers are made are called monomers. Other alkenes also undergo polymerisation.
 (13.53)
(13.53)
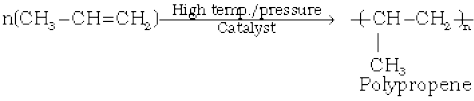 (13.54)
(13.54)
Polymers are used for the manufacture of plastic bags, squeeze bottles, refrigerator dishes, toys, pipes, radio and T.V. cabinets etc. Polypropene is used for the manufacture of milk crates, plastic buckets and other moulded articles. Though these materials have now become common, excessive use of polythene and polypropylene is a matter of great concern for all of us.