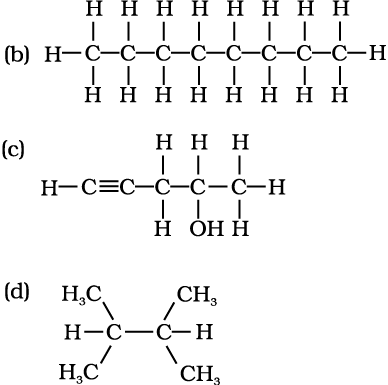
Structures of organic compounds are represented in several ways. The Lewis structure or dot structure, dash structure, condensed structure and bond line structural formulas are some of the specific types. The Lewis structures, however, can be simplified by representing the two-electron covalent bond by a dash (–). Such a structural formula focuses on the electrons involved in bond formation. A single dash represents a single bond, double dash is used for double bond and a triple dash represents triple bond. Lone-pairs of electrons on heteroatoms (e.g., oxygen, nitrogen, sulphur, halogens etc.) may or may not be shown. Thus, ethane (C2H6), ethene (C2H4), ethyne (C2H2) and methanol (CH3OH) can be represented by the following structural formulas. Such structural representations are called complete structural formulas.
These structural formulas can be further abbreviated by omitting some or all of the dashes representing covalent bonds and by indicating the number of identical groups attached to an atom by a subscript. The resulting expression of the compound is called a condensed structural formula. Thus, ethane, ethene, ethyne and methanol can be written as:
CH3CH3 H2C=CH2 HC≡CH CH3OH
Ethane Ethene Ethyne Methanol
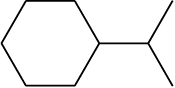
Similarly, CH3CH2CH2CH2CH2CH2CH2CH3 can be further condensed to CH3(CH2)6CH3. For further simplification, organic chemists use another way of representing the structures, in which only lines are used. In this bond-line structural representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The terminals denote methyl (–CH3) groups (unless indicated otherwise by a functional group), while the line junctions denote carbon atoms bonded to appropriate number of hydrogens required to satisfy the valency of the carbon atoms. Some of the examples are represented as follows:
(i) 3-Methyloctane can be represented in various forms as:
(a)
(b)
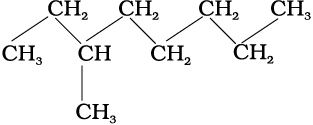
(c)
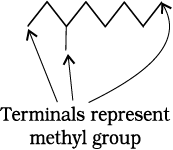
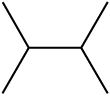
(ii) Various ways of representing 2-bromo butane are:
(a) CH3CHBrCH2CH3
(b)
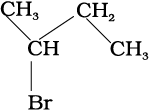
(c)
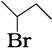
In cyclic compounds, the bond-line formulas may be given as follows:
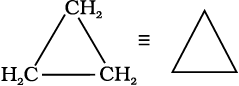
Cyclopropane
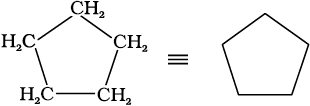
Cyclopentane
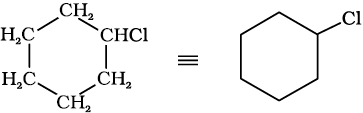
chlorocyclohexane
Problem 12.4
Expand each of the following condensed formulas into their complete structural formulas.
(a) CH3CH2COCH2CH3
(b) CH3CH=CH(CH2)3CH3
Solution
(a)
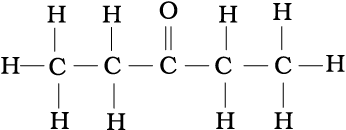
(b)
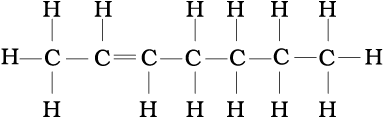
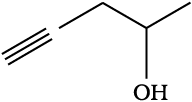
Problem 12.5
For each of the following compounds, write a condensed formula and also their bond-line formula.
(a) HOCH2CH2CH2CH(CH3)CH(CH3)CH3
(b)
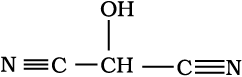
Solution
Condensed formula:
(a) HO(CH2)3CH(CH3)CH(CH3)2
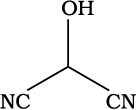
(b) HOCH(CN)2
Bond-line formula:
(a)
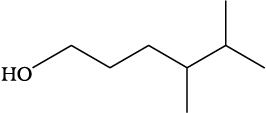
(b)
Problem 12.6
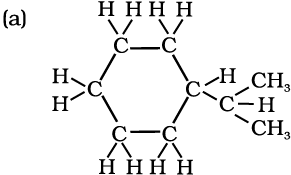
Expand each of the following bond-line formulas to show all the atoms including carbon and hydrogen
(a)