Boron is non-metallic in nature. It is extremely hard and black coloured solid. It exists in many allotropic forms. Due to very strong crystalline lattice, boron has unusually high melting point. Rest of the members are soft metals with low melting point and high electrical conductivity. It is worthwhile to note that gallium with unusually low melting point (303K), could exist in liquid state during summer. Its high boiling point (2676K) makes it a useful material for measuring high temperatures. Density of the elements increases down the group from boron to thallium.
Due to small size of boron, the sum of its first three ionization enthalpies is very high. This prevents it to form +3 ions and forces it to form only covalent compounds. But as we move from B to Al, the sum of the first three ionisation enthalpies of Al considerably decreases, and is therefore able to form Al3+ ions. In fact, aluminium is a highly electropositive metal. However, down the group, due to poor shielding effect of intervening d and f orbitals, the increased effective nuclear charge holds ns electrons tightly (responsible for inert pair effect) and thereby, restricting their participation in bonding. As a result of this, only p-orbital electron may be involved in bonding. In fact in Ga, In and Tl, both +1 and +3 oxidation states are observed. The relative stability of +1 oxidation state progressively increases for heavier elements: Al
Table 11.2 Atomic and Physical Properties of Group 13 Elements
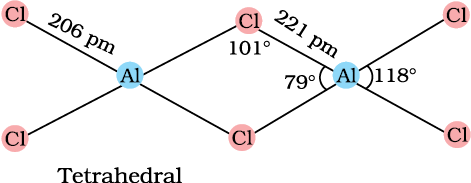
AlCl3 achieves stability by forming a dimer
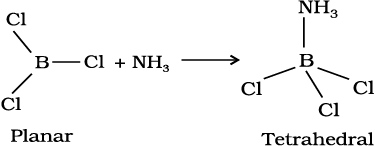
In trivalent state most of the compounds being covalent are hydrolysed in water. For example, the trichlorides on hyrolysis in water form tetrahedral 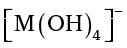 species; the hybridisation state of element M is sp3. Aluminium chloride in acidified aqueous solution forms octahedral
species; the hybridisation state of element M is sp3. Aluminium chloride in acidified aqueous solution forms octahedral 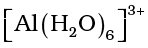 ion. In this complex ion, the 3d orbitals of Al are involved and the hybridisation state of Al is sp3d2.
ion. In this complex ion, the 3d orbitals of Al are involved and the hybridisation state of Al is sp3d2.
Problem 11.1
Standard electrode potential values, E for Al3+/Al is –1.66 V and that of Tl3+/Tl is +1.26 V. Predict about the formation of M3+ ion in solution and compare the electropositive character of the two
metals.
Solution
Standard electrode potential values for two half cell reactions suggest that aluminium has high tendency to make Al3+(aq) ions, whereas Tl3+ is not only unstable in solution but is a powerful oxidising agent also. Thus Tl+ is more stable in solution than Tl3+. Aluminium being able to form +3 ions easily, is more electropositive than thallium.