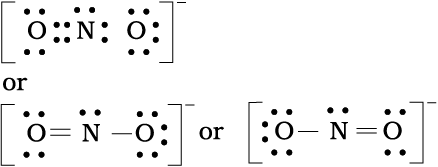Langmuir (1919) refined the Lewis postulations by abandoning the idea of the stationary cubical arrangement of the octet, and by introducing the term covalent bond. The Lewis-Langmuir theory can be understood by considering the formation of the chlorine molecule,Cl2. The Cl atom with electronic configuration, [Ne]3s2 3p5, is one electron short of the argon configuration. The formation of the Cl2 molecule can be understood in terms of the sharing of a pair of electrons between the two chlorine atoms, each chlorine atom contributing one electron to the shared pair. In the process both chlorine atoms attain the outer shell octet of the nearest noble gas (i.e., argon).
or Cl – Cl
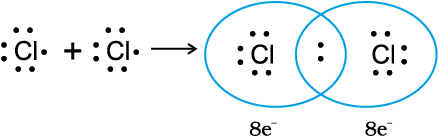
The dots represent electrons. Such structures are referred to as Lewis dot structures.
The Lewis dot structures can be written for other molecules also, in which the combining atoms may be identical or different. The important conditions being that:
• Each bond is formed as a result of sharing of an electron pair between the atoms.
• Each combining atom contributes at least one electron to the shared pair.
• The combining atoms attain the outer-shell noble gas configurations as a result of the sharing of electrons.
• Thus in water and carbon tetrachloride molecules, formation of covalent bonds can be represented as:
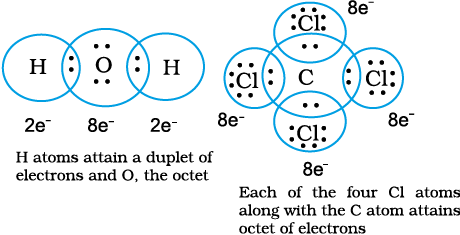
Thus, when two atoms share one electron pair they are said to be joined by a single covalent bond. In many compounds we have multiple bonds between atoms. The formation of multiple bonds envisages sharing of more than one electron pair between two atoms. If two atoms share two pairs of electrons, the covalent bond between them is called a double bond. For example, in the carbon dioxide molecule, we have two double bonds between the carbon and oxygen atoms. Similarly in ethene molecule the two carbon atoms are joined by a double bond.
When combining atoms share three electron pairs as in the case of two nitrogen atoms in the N2 molecule and the two carbon atoms in the ethyne molecule, a triple bond is formed.
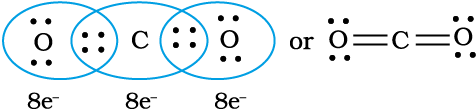
Double bonds in CO2 molecule
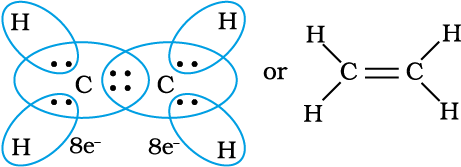
C2H4 molecule
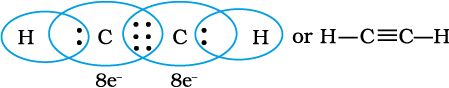
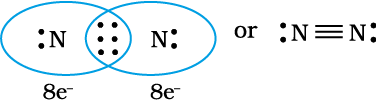
C2H2 molecule
4.1.3 Lewis Representation of Simple Molecules (the Lewis Structures)
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule. While such a picture may not explain the bonding and behaviour of a molecule completely, it does help in understanding the formation and properties of a molecule to a large extent. Writing of Lewis dot structures of molecules is, therefore, very useful. The Lewis dot structures can be written by adopting the following steps:
• The total number of electrons required for writing the structures are obtained by adding the valence electrons of the combining atoms. For example, in the CH4 molecule there are eight valence electrons available for bonding (4 from carbon and 4 from the four hydrogen atoms).
• For anions, each negative charge would mean addition of one electron. For cations, each positive charge would result in subtraction of one electron from the total number of valence electrons. For example, for the CO32– ion, the two negative charges indicate that there are two additional electrons than those provided by the neutral atoms. For NH4+ ion, one positive charge indicates the loss of one electron from the group of neutral atoms.
• Knowing the chemical symbols of the combining atoms and having knowledge of the skeletal structure of the compound (known or guessed intelligently), it is easy to distribute the total number of electrons as bonding shared pairs between the atoms in proportion to the total bonds.
• In general the least electronegative atom occupies the central position in the molecule/ion. For example in the NF3 and CO32–, nitrogen and carbon are the central atoms whereas fluorine and oxygen occupy the terminal positions.
• After accounting for the shared pairs of electrons for single bonds, the remaining electron pairs are either utilized for multiple bonding or remain as the lone pairs. The basic requirement being that each bonded atom gets an octet of electrons.
Lewis representations of a few molecules/ ions are given in Table 4.1.
Table 4.1 The Lewis Representation of Some Molecules
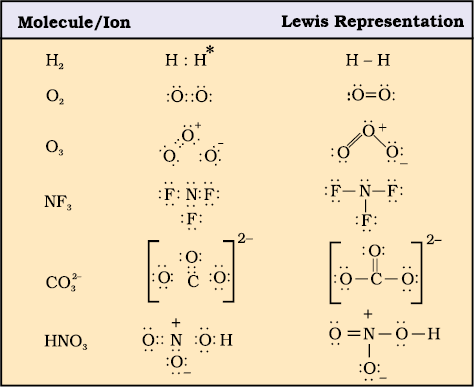
* Each H atom attains the configuration of helium (a duplet of electrons)
Problem 4.1
Write the Lewis dot structure of CO molecule.
Solution
Step 1. Count the total number of valence electrons of carbon and oxygen atoms. The outer (valence) shell configurations of carbon and oxygen atoms are: 2s2 2p2 and 2s2 2p4, respectively. The valence electrons available are 4 + 6 =10.
Step 2. The skeletal structure of CO is written as: C O
Step 3. Draw a single bond (one shared electron pair) between C and O and complete the octet on O, the remaining two electrons are the lone pair on C.
This does not complete the octet on carbon and hence we have to resort to multiple bonding (in this case a triple bond) between C and O atoms. This satisfies the octet rule condition for both atoms.

Problem 4.2
Write the Lewis structure of the nitrite ion, NO2– .
Solution
Step 1. Count the total number of valence electrons of the nitrogen atom, the oxygen atoms and the additional one negative charge (equal to one electron).
N(2s2 2p3), O (2s2 2p4)
5 + (2 × 6) +1 = 18 electrons
Step 2. The skeletal structure of NO2– is written as : O N O
Step 3. Draw a single bond (one shared electron pair) between the nitrogen and each of the oxygen atoms completing the octets on oxygen atoms. This, however, does not complete the octet on nitrogen if the remaining two electrons constitute lone pair on it.
Hence we have to resort to multiple bonding between nitrogen and one of the oxygen atoms (in this case a double bond). This leads to the following Lewis dot structures.