The combination of elements to form compounds is governed by the following five basic laws.

Antoine Lavoisier
(1743–1794)
It states that matter can neither be created nor destroyed.
This law was put forth by Antoine Lavoisier in 1789. He performed careful experimental studies for combustion reactions and reached to the conclusion that in all physical and chemical changes, there is no net change in mass duting the process. Hence, he reached to the conclusion that matter can neither be created nor destroyed. This is called ‘Law of Conservation of Mass’. This law formed the basis for several later developments in chemistry. Infact, this was the result of exact measurement of masses of reactants and products, and carefully planned experiments performed by Lavoisier.

Joseph Proust
(1754–1826)
This law was given by, a French chemist, Joseph Proust. He stated that a given compound always contains exactly the same proportion of elements by weight.
Proust worked with two samples of cupric carbonate — one of which was of natural origin and the other was synthetic. He found that the composition of elements present in it was same for both the samples as shown below:
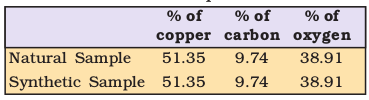
Thus, he concluded that irrespective of the source, a given compound always contains same elements combined together in the same proportion by mass. The validity of this law has been confirmed by various experiments. It is sometimes also referred to as Law of Definite Composition.
This law was proposed by Dalton in 1803. According to this law, if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
For example, hydrogen combines with oxygen to form two compounds, namely, water and hydrogen peroxide.
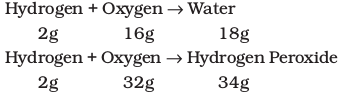
Here, the masses of oxygen (i.e., 16 g and 32 g), which combine with a fixed mass of hydrogen (2g) bear a simple ratio, i.e., 16:32 or 1: 2.

Joseph Louis
Gay Lussac

© 2026 GoodEd Technologies Pvt. Ltd.