Volume is the amont of space occupied by a substance. It has the units of (length)3. So in SI system, volume has units of m3. But again, in chemistry laboratories, smaller volumes are used. Hence, volume is often denoted in cm3 or dm3 units.
Fig. 1.6 helps to visualise these relations.
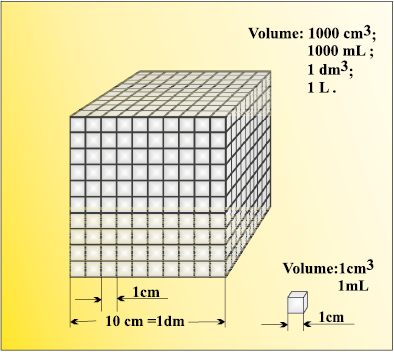
Fig. 1.6 Different units used to express volume
A common unit, litre (L) which is not an SI unit, is used for measurement of volume of liquids.
1 L = 1000 mL , 1000 cm3 = 1 dm3
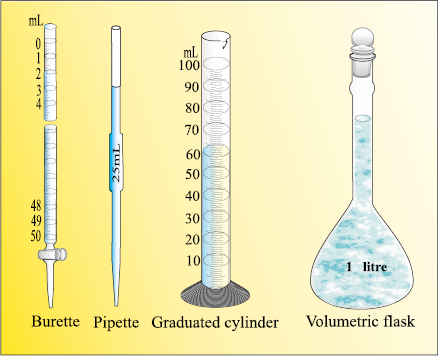
Fig. 1.7 Some volume measuring devices
In the laboratory, the volume of liquids or solutions can be measured by graduated cylinder, burette, pipette, etc. A volumetric flask is used to prepare a known volume of a solution. These measuring devices are shown in Fig. 1.7.
The two properties — mass and volume discussed above are related as follows:
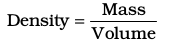
Density of a substance is its amount of mass per unit volume. So, SI units of density can be obtained as follows:
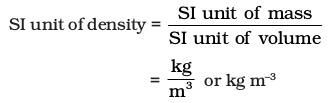
This unit is quite large and a chemist often expresses density in g cm–3, where mass is expressed in gram and volume is expressed in cm3. Density of a substance tells us about how closely its particles are packed. If density is more, it means particles are more closely packed.

© 2026 GoodEd Technologies Pvt. Ltd.