4.8 In a pseudo first order reaction in water, the following results were obtained:
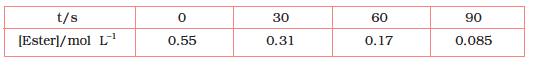
(i) Calculate the average rate of reaction between the time interval 30 to 60 seconds.
(ii) Calculate the pseudo first order rate constant for the hydrolysis of ester.
(i) Average rate of reaction between the time interval, 30 to 60 seconds,
=
(ii) For a pseudo first order reaction,

© 2026 GoodEd Technologies Pvt. Ltd.