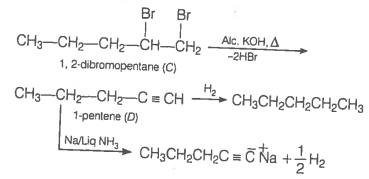
An alkyl halide reacts with ethanolic KOH to give an alkene 'B', which reacts with Br2 to give a compound 'C', which on dehydrobromination gives an alkyne 'D'. On treatment with sodium metal in liquid ammonia, one mole of 'D' gives one mole of the sodium salt of 'D' and half a mole of hydrogen gas. Complete hydrogenation of 'D' yields a straight chain alkane. Identify A, B, C and D. Give the reactions involved.

© 2026 GoodEd Technologies Pvt. Ltd.