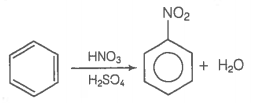
| Assertion (A): | Nitration of benzene with nitric acid requires the use of concentrated sulphuric acid. |
| Reason (R): | The mixture of concentrated sulphuric acid and concentrated nitric acid produces the electrophile, NO2+ . |
| 1. | Both (A) and (R) are True and (R) is the correct explanation of (A). |
| 2. | Both (A) and (R) are True but (R) is not the correct explanation of (A). |
| 3. | (A) is True but (R) is False. |
| 4. | (A) is False but (R) is True. |

© 2026 GoodEd Technologies Pvt. Ltd.