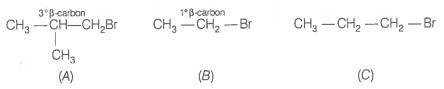
Arrange the following alkyl halides in decreasing order of the rate of -elimination reaction with alcoholic KOH.
| A. | \(\mathrm{(CH_3)_2C(H)CH_2Br}\) |
| B. | CH3-CH2-Br |
| C. | CH3-CH2-CH2-Br |
1. A > B > C
2. C > B > A
3. B > C > A
4. A > C > B
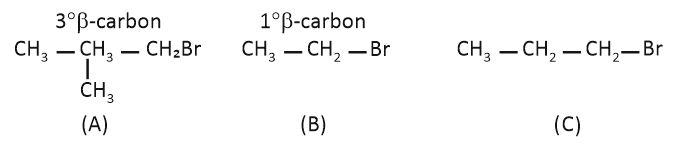

© 2026 GoodEd Technologies Pvt. Ltd.