Write the reactions of D-glucose which can't be explained by its open-chain structure. How can the cyclic structure of glucose explain these reactions?
Chemical reactions of D-glucose which can't be explained by its open-chain structure are
(i) Glucose does not give Schiff's test and does not produce hydrogen sulfite addition products with NaHso3. despite having an aldehyde group
(ii) The pentaacetate of glucose does not react with hydroxylamine.
In actuality, glucose exists in two different crystalline form forms and ß form. It was proposed that one of the OH groups may add to the the-CHO group and form a cyclic hemiacetal structure. Glucose forms a 6 membered pyranose structure.
Cyclic strUcture exist in equilibrium with an open structure and can be represented as
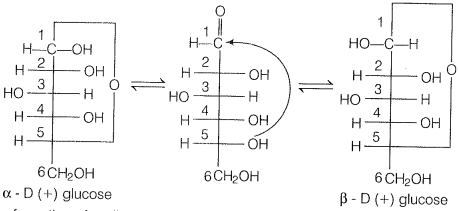
Due to the formation of the cyclic structure of glucose CHO group of glucose remain no longer free due to which they do not show the above-given reactions.

© 2026 GoodEd Technologies Pvt. Ltd.