16.21 Explain the following terms with suitable examples
(i) cationic detergents
(ii) anionic detergents and
(iii) non-ionic detergents.
(i) Cationic detergent
Cationic detergents are quaternary ammonium salts of acetates, chlorides, or bromides. These are called cationic detergents because the cationic part of these detergents contains a long hydrocarbon chain and a positive charge on the N atom. For example: Cetyltrimethylammonium bromide
(ii) Anionic detergents
Anionic detergents are of two types:
1.Sodium alkyl sulphates:
These detergents are sodium salts of long chain alcohols. They are prepared by first treating these alcohols with concentrated sulphuric acid and then with sodium hydroxide. Examples of these detergents include sodium lauryl sulphate () and sodium stearyl sulphate
().
2. Sodium alkylbenzenesulphonates:
These detergents are sodium salts of long chain alkylbenzenesulphonic acids. They are prepared by Friedel-Crafts alkylation of benzene with long chain alkyl halides or alkenes. The obtained product is first treated with concentrated sulphuric acid and then with sodium hydroxide. Sodium 4-(1-dodecy) benzenesulphonate (SDS) is an example of anionic detergents.
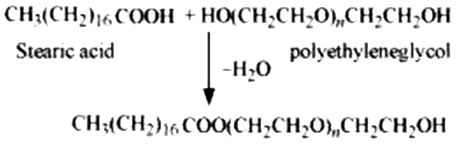
(iii) Non-ionic detergents
Molecules of these detergents do not contain any ions. These detergents are esters of alcohols having high molecular mass. They are obtained by reacting polyethylene glycol and stearic acid.

© 2026 GoodEd Technologies Pvt. Ltd.