What is the side product of the soap industry? Give reactions showing soap formation.
Soaps are sodium or potassium salts of long-chain faulty acids such as stearic acid, oleic acid, and palmitic acid. Soaps containing sodium salts are formed by heating fat (i.e., glyceryl ester of fatty acid) with aqueous sodium hydroxide Ans.
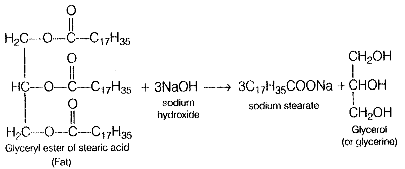
This reaction is known as saponification. In this reaction, esters of fatty acids are hydrolyzed and the soap obtained remains in colloidal form. It is precipitated from the solution by adding NaCI. The solution left after removing the soap contains glycerol as a side product.

© 2026 GoodEd Technologies Pvt. Ltd.