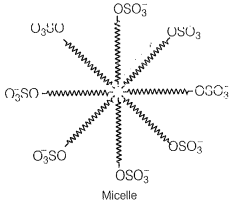
Draw the diagram showing micelle formation by the following detergent. , Na
Sodium lauryl sulfate, Na is an example of anionic detergent.
When added to water, it dissociates as follows
These anions are present on the surface with their- OSO groups in water and hydrocarbon part staying away from it and remain at the surface
At higher concentrations, these anions are pulled into the bulk of the solution and form an aggregate of spherical shape with their hydrocarbon part pointing towards the center and part outwards on the surface of the sphere.
An aggravate thus formed is known as a micelle.

© 2026 GoodEd Technologies Pvt. Ltd.