If the soap has high alkali content it irritates the skin. How can the amount of excess alkali be determined? What can be the source of excess alkali?
A solution of soap is titrated with standard hydrochloric acid. It is an acid-base titration. In this titration, phenolphthalein is used as an indicator. During the preparation of soap, fat i.e., glyceryl ester of fatty acid) is heated with aqueous sodium hydroxide
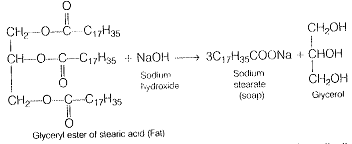
Thus, the source of this excess alkali (which irritates the skin) is the alkali left unused when the Soap is prepared by hydrolysis of fat.

© 2026 GoodEd Technologies Pvt. Ltd.