Question 15.8:
How can you differentiate between addition and condensation polymerization?
Addition polymerization is the process of repeated addition of monomers, possessing double or triple bonds to form polymers. For example, polythene is formed by the addition polymerization of ethene.
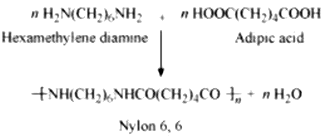
Condensation polymerization is the process of formation of polymers by repeated condensation reactions between two different bi-functional or tri-functional monomers. A small molecule such as water or hydrochloric acid is eliminated in each condensation. For example, nylon 6, 6 is formed by condensation polymerization of hexamethylenediamine and adipic acid.

© 2026 GoodEd Technologies Pvt. Ltd.