13.14 Give plausible explanation for each of the following:
(i) Why are amines less acidic than alcohols of comparable molecular masses?
(ii) Why do primary amines have higher boiling point than tertiary amines?
(iii) Why are aliphatic amines stronger bases than aromatic amines?
(i)
Acidity depends on the stability of the conjugate base. In the case of amine, the negative charge is on the N-atom whereas, an alkoxide ion, the negative charge is on the O-atom.
Since O is more electronegative than N, O can accommodate the negative charge more easily than N.
As a result, the amide ion is less stable than the alkoxide ion. Hence, amines are less acidic than alcohols of comparable molecular masses.
(ii)
In a molecule of a tertiary amine, there are no H−atoms whereas in primary amines, two hydrogen atoms are present. Due to the presence of H−atoms, primary amines undergo extensive intermolecular H−bonding.
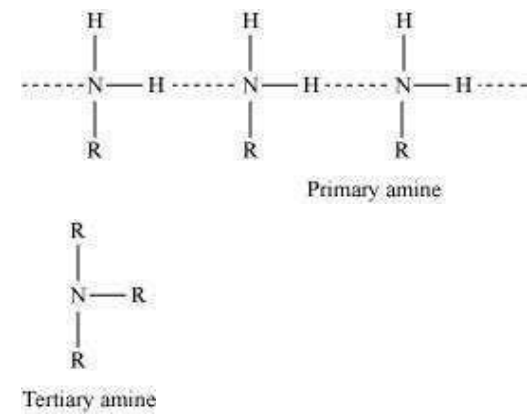
As a result, extra energy is required to separate the molecules of primary amines. Hence, primary amines have higher boiling points than tertiary amines.
(iii)
Due to the −R effect of the benzene ring, the electrons on the N- atom are less available in the case of aromatic amines. Therefore, the electrons on the N-atom in aromatic amines cannot be donated easily.
But in the case of aliphatic amines, +I effect of the alkyl group increases the electron density in the nitrogen atom This explains why aliphatic amines are stronger bases than aromatic amines.

© 2026 GoodEd Technologies Pvt. Ltd.