13.12 Why cannot aromatic primary amines be prepared by Gabriel phthalimide synthesis?
Gabriel phthalimide synthesis is used for the preparation of aliphatic primary amines. It involves nucleophilic substitution (SN2) of alkyl halides by the anion formed by the phthalimide.
But aryl halides do not undergo nucleophilic substitution with the anion formed by the phthalimide.
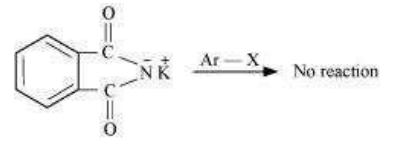
Hence, aromatic primary amines cannot be prepared by this process.

© 2026 GoodEd Technologies Pvt. Ltd.
