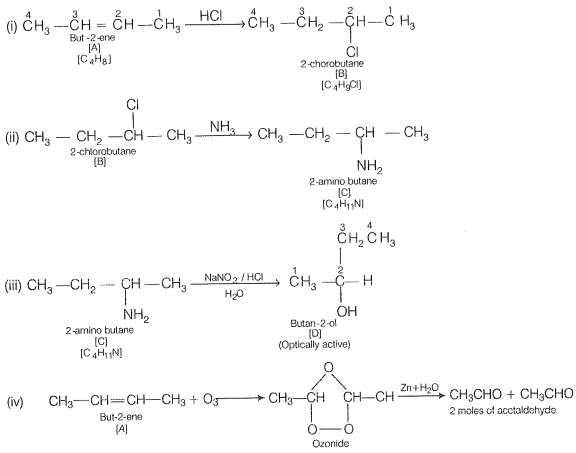
Q.75 A hydrocarbon 'A' (C4H8) on reaction with HCI gives a compound 'B,' (C4H9Cl), which on reaction with 1 mol of NH3 gives compound 'C,' (C4H11N). On reacting with NaNO2 and HCI followed by treatment with water, compound 'C' yields optically active alcohol, 'D'. Ozonolysis of 'A' gives 2 mols of acetaldehyde. Identify compounds 'A' to 'D'. Explain the reactions involved.
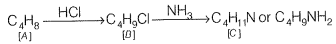

© 2026 GoodEd Technologies Pvt. Ltd.