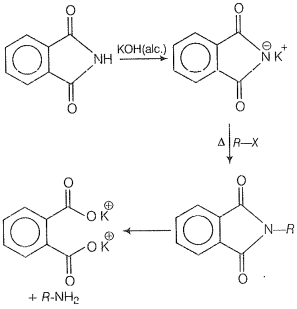
Which of the following methods of preparation of amines will not give the same number of carbon atoms in the chain of amines as in the reactant?
| 1. | Reaction of nitrile with LiAlH4 |
| 2. | Reaction of amide with LiAlH4 followed by treatment with water |
| 3. | Heating alkyl halide with potassium salt of phthalimide followed by hydrolysis |
| 4. | Treatment of amide with bromine in an aqueous solution of sodium hydroxide. |




© 2026 GoodEd Technologies Pvt. Ltd.