12.17 Complete each synthesis by giving missing starting material, reagent, or products
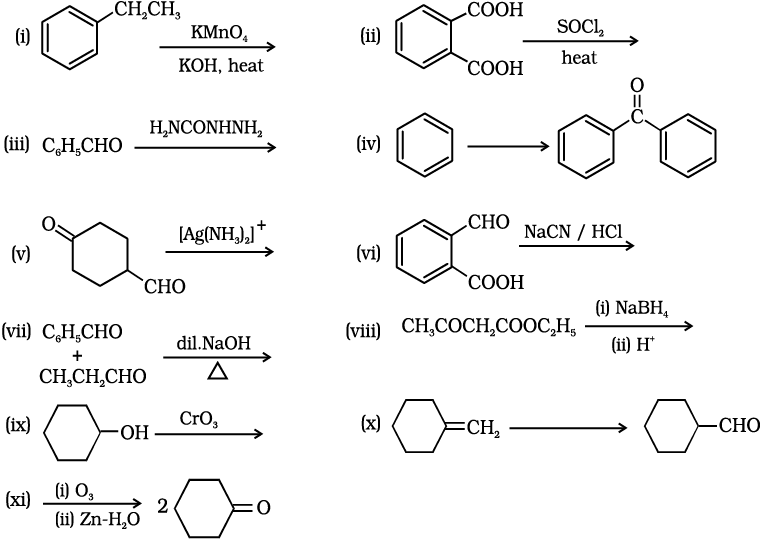
(i)
This reaction is an example of an oxidation reaction. The ethyl group gets oxidized and potassium benzoate is formed as a product. The reaction is as follows:
(ii)
This reaction is an example of a nucleophilic substitution reaction. The OH group in the acid is substituted by Cl atom when acyl chloride reacts with SOCl2 . The reaction is as follows:
(iii)
When benzaldehyde reacts with semicarbazide then semicarbazone is formed as a product. The reaction is as follows:
(iv)
In this reaction, benzene reacts with benzoyl chloride in the presence of anhydride. AlCl3 and benzophenone is obtained as a product.
The reaction is as follows:
(v)
The given compound contains both aldehyde and ketone groups, hence in the presence of a mild oxidizing agent, only the aldehyde group gets oxidized not ketone. Thus, when the given compound reacts with tollens' reagent then the CHO group converts into the carboxylate group. The reaction is as follows:
(vi)
In this reaction, the -CHO group present in the compound reacts with NaCN in the acidic medium, and cyanohydrin is obtained as a product.
The reaction is as follows:
(vii)
In this reaction, benzaldehyde reacts with propanal in the presence of a base followed by heating then an aldol condensation reaction occurs, and α,β-Unsaturated aldehydes are obtained. The reaction is as follows:
(viii)
The given compound contains both ketone and an ester group hence when such compound reacts with NaBH4 then selectively ketone group gets reduced and the ester group does not.
The reaction is as follows:
(ix)
When secondary alcohol reacts with an oxidizing agent then ketone is formed as a product. The CrO3-H2SO4 is an oxidizing agent. The reaction is as follows:
(x)
The given transformation can be achieved in two steps. In the first step, hydroboration and oxidation reactions occur. When alkene reacts with BH3 followed by H2O2/OH- then alcohol is obtained as a product.
In the second step, oxidation of -OH group takes place and converts into the -CHO group. The reagent used for this reaction is PCC.
The reactions are as follows:
(xi)
When the given compound reacts with ozone followed by Zn-H2O then reductive ozonolysis takes place and ketone is obtained as a product.
The reaction is as follows: