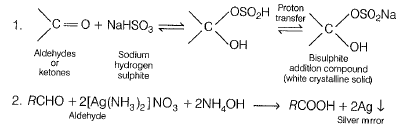
When liquid ‘A’ is treated with a freshly prepared ammoniacal silver nitrate solution it gives bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogen sulphite. Liquid ‘B’ also forms a white crystalline solid with sodium hydrogen sulphite but it does not give test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.

© 2026 GoodEd Technologies Pvt. Ltd.