Write down functional isomers of a carbonyl compound with molecular formula . Which isomer will react faster with HCN and why? Explain the mechanism of the reaction also. Will the reaction lead to the completion with the conversion uf whole reactant into product at reaction conditions? If a strong acid is added to the reaction mixture what will be the effect on concentration of the product and why?
(a) Propanal, will react faster with HCN because there is less steric hindrance and electronic factors, which increases its electrophilicity.
(b) The reaction mechanism is as follow
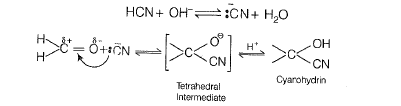
The reaction does not lead to completion because it is a reversible reaction. Equilibrium is established.
(c) It a strong acid is added to the reaction mixture, the reaction is inhibited because production of CN ions prevented.

© 2026 GoodEd Technologies Pvt. Ltd.