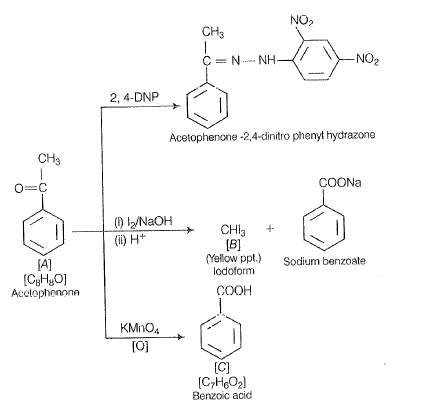
An aromatic compound ‘4’ (molecular formula) gives positive 2, 4-DNP test. It gives a yellow precipitate of compound ‘8’ on treatment with iodine and sodium hydroxide solution. Compound ‘A’ does not give Tollen's or Fehling's test. On drastic oxidation with potassium Permanganate it forms a carboxylic acid ‘C’ (molecular formula ), which is also formed along with the yellow compound in the above reaction. Identify A, B, and C and write all the reactions involved.
Degree of unsaturation =
Degree of unsaturation > 5 ie., it may contain benzene ring having degree of unsaturation equal to 4 and one degree of unsaturation must be carbonyl group.
Thus, possible structures are
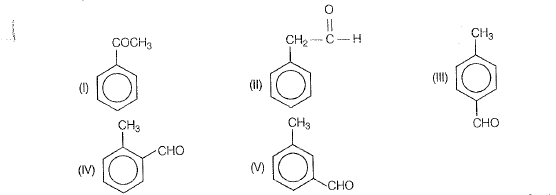
‘According to question, compound ‘A’ does not respond to Tollen's or Fehling's test, So, itis a ketone not aldehyde. Therefore, structure | is correct. Complete reaction sequence is as follows

© 2026 GoodEd Technologies Pvt. Ltd.