Alkenes 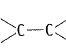 and carbonyl compounds
and carbonyl compounds 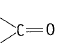 both contain a bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.
both contain a bond but alkenes show electrophilic addition reactions whereas carbonyl compounds show nucleophilic addition reactions. Explain.

© 2026 GoodEd Technologies Pvt. Ltd.