Why is there a large difference in the boiling points of butanal and butan-1-ol?
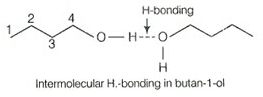
Bulanol has polar O—H bond due to which it shows intermolecular H-bonding which is not possible in case of butanal due to absence of polar bond. Instead of it has only weak dipole-dipole interactions. Hence, bulanal has higher boiling point than butan-1-ol.

© 2026 GoodEd Technologies Pvt. Ltd.