12.1 What is meant by the following terms ? Give an example of the reaction in each case.
(i) Cyanohydrin (ii) Acetal (iii) Semicarbazone
(iv) Aldol (v) Hemiacetal (vi) Oxime
(vii) Ketal (vii) Imine (ix) 2,4-DNP-derivative
(x) Schiff’s base
(i)
Step 1:
Cyanohydrin:
Cyanohydrins are organic compounds having the formula RR′C(OH)CN, where R and R′ can be alkyl or aryl groups.
The structure is as follows:
Step 2:
Aldehydes and ketones react with hydrogen cyanide (HCN) in the presence of excess sodium cyanide (NaCN) as a catalyst to form cyanohydrin. The reaction follows the nucleophilic addition reaction mechanism.
The reaction is as follows:
(ii)
Step 1:
Acetal:
Aldehydes react with an excess of monohydric alcohol in the presence of dry hydrogen chloride to give a gem-alkoxy compound known as acetal. The structure is as follows:
Step 2:
The reaction is as follows:
(iii)
Step 1:
Semicarbarbazone:
When nucleophiles, such as semicarbazide (derivatives of ammonia) add to the carbonyl group of aldehydes and ketones, then Semicarbazones product is obtained as a product. The product is >C=N-Z. In the case of semicarbazone, Z is NHCONH2.
The general reaction for aldehyde and ketone is as follows:
Semicarbazones are useful for the identification and characterization of aldehydes and ketones.
(iv)
Step 1:
Aldol:
A β-hydroxy aldehyde or ketone is known as an aldol. When aldehydes and ketones having at least one α-hydrogen undergo a reaction in the presence of dilute alkali as a catalyst then β-hydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively are obtained as the product. This reaction is known as the Aldol reaction.
Step 2:
The reaction is as follows:
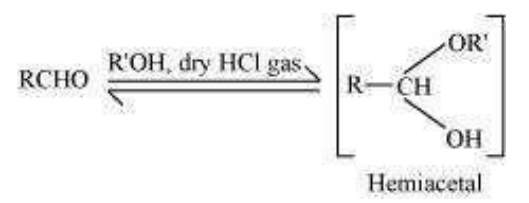
(v)
Step 1:
Hemiacetal:
Hemiacetals are α−alkoxyalcohols.
Aldehydes react with one equivalent of monohydric alcohol in the presence of dry hydrogen chloride to yield alkoxyalcohol intermediate, known as hemiacetals. The structure is as follows:
The reaction is as follows:
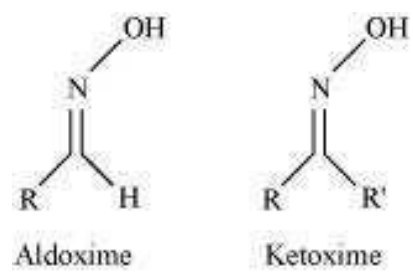
(vi)
Step 1:
Oxime:
Nucleophiles, such as NH2OH(derivative of ammonia) react with the carbonyl group of aldehydes and ketones then oxime is obtained as a product.
When aldehyde forms oxime it is known as aldoxime, if ketone forms oxime then it is called ketoxime.
The structure is as follows:
Step 2:
The reaction is as follows:

(vii)
Step 1:
Ketal:
Ketals are gem−dialkoxyalkanes in which two alkoxy groups are present on the same carbon atom within the chain. The structure is as follows:
Step 2:
When ketone reacts with an excess of monohydric alcohol in the presence of dry hydrogen chloride to yield gem−dialkoxyalkanes, is known as ketal. The reaction is as follows:
(viii)
Step 1:
Imine:
Imines are chemical compounds containing a carbon-nitrogen double bond. When aldehyde or ketone reacts with ammonia then imine is formed as a product. The general structure of an imine is as follows:
Step 2:
The reaction is as follows:
(ix)
Step 1:
2, 4−DNP derivative:
2,4−Dinitrophenylhydrazones are 2, 4−DNP−derivatives, which are produced when aldehydes or ketones react with
2, 4−dinitrophenylhydrazine in a weakly acidic medium. The general structure is as follows:
Step 2:
The reaction is as follows:
(x)
Step 1:
Schiff’s base:
Schiff’s base (or azomethine) is a chemical compound containing a carbon-nitrogen double bond with the nitrogen atom connected to an aryl or alkyl group-but, not hydrogen.
When aldehyde or ketone reacts with an amine in the presence of a trace of acid then Schiff's base is obtained as a product.
The structure is as follows:
The reaction is as follows: