11.25 Illustrate with examples the limitations of Williamson synthesis for the preparation of certain types of ethers.
The reaction of Williamson synthesis involves SN2 attack of an alkoxide ion on a primary
alkyl halide.
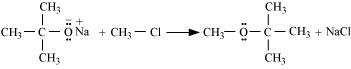
But if secondary or tertiary alkyl halides are taken in place of primary alkyl halides, then
elimination would compete over substitution. As a result, alkenes would be produced. This
is because alkoxides are nucleophiles as well as strong bases. Hence, they react with alkyl
halides, which results in an elimination reaction.
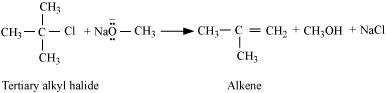

© 2026 GoodEd Technologies Pvt. Ltd.