11.5 Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Alcohols form H-bonds with water due to the presence of −OH group. However,
hydrocarbons cannot form H-bonds with water.
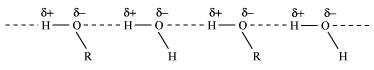
As a result, alcohols are comparatively more soluble in water than hydrocarbons of
comparable molecular masses.

© 2026 GoodEd Technologies Pvt. Ltd.