11.4 Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Propanol undergoes intermolecular H-bonding because of the presence of −OH group. On
the other hand, butane does not
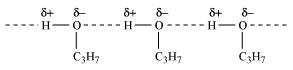
Therefore, extra energy is required to break hydrogen bonds. For this reason, propanol
has a higher boiling point than hydrocarbon butane.

© 2026 GoodEd Technologies Pvt. Ltd.