11.22 Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
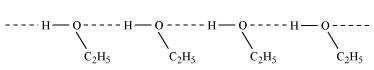
Ethanol undergoes intermolecular H-bonding due to the presence of −OH group, resulting
in the association of molecules. Extra energy is required to break these hydrogen bonds.
On the other hand, methoxymethane does not undergo H-bonding.
Hence, the boiling point of ethanol is higher than that of methoxymethane.

© 2026 GoodEd Technologies Pvt. Ltd.