11.19 Write the mechanism of acid dehydration of ethanol to yield ethene.
The mechanism of acid dehydration of ethanol to yield ethene involves the following three
steps:
Step 1:
Protonation of ethanol to form ethyl oxonium ion:
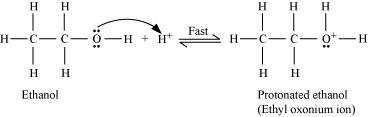
Step 2:
Formation of carbocation (rate determining step):
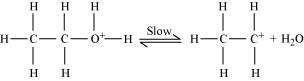
Step 3:
Elimination of a proton to form ethene: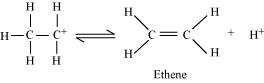
The acid consumed in step 1 is released in Step 3. After the formation of ethene, it is
removed to shift the equilibrium in a forward direction.

© 2026 GoodEd Technologies Pvt. Ltd.