11.18 Explain the following with an example.
(i) Kolbe’s reaction.
(ii) Reimer-Tiemann reaction.
(iii) Williamson ether synthesis.
(iv) Unsymmetrical ether.
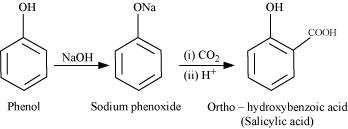
(i) Kolbe’s reaction:
When phenol is treated with sodium hydroxide, sodium phenoxide is produced. This sodium
phenoxide when treated with carbon dioxide, followed by acidification, undergoes
electrophilic substitution to give ortho-hydroxybenzoic acid as the main product. This
reaction is known as Kolbe’s reaction.
(ii) Reimer-Tiemann reaction:
When phenol is treated with chloroform (CHCl3) in the presence of sodium hydroxide, a
−CHO group is introduced at the ortho position of the benzene ring.
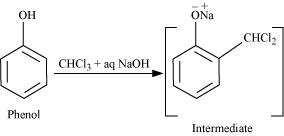
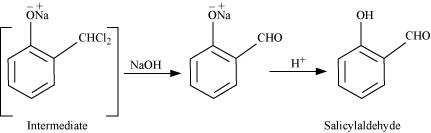
This reaction is known as the Reimer-Tiemann reaction.
The intermediate is hydrolyzed in the presence of alkalis to produce salicyclaldehyde.
(iii) Williamson ether synthesis:
Williamson ether synthesis is a laboratory method to prepare symmetrical and
unsymmetrical ethers by allowing alkyl halides to react with sodium alkoxides.
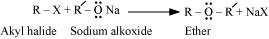
This reaction involves SN2 attack of the alkoxide ion on the alkyl halide. Better results are
obtained in case of primary alkyl halides.
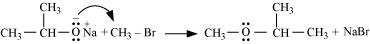
If the alkyl halide is secondary or tertiary, then elimination competes over substitution.
(iv) Unsymmetrical ether:
An unsymmetrical ether is an ether where two groups on the two sides of an oxygen atom
differ (i.e., have an unequal number of carbon atoms). For example: ethyl methyl ether
(CH−O−CHCH).

© 2026 GoodEd Technologies Pvt. Ltd.