The mechanism of hydration of ethene to form ethanol involves three steps.
Step 1:
Protonation of ethene to form carbocation by electrophilic attack of H3O+:
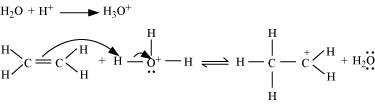
Step 2:
Nucleophilic attack of water on carbocation:
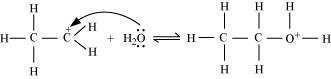
Step 3:
Deprotonation to form ethanol:
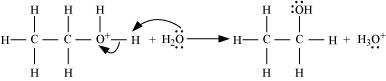
11.12 You are given benzene, conc. H2SO4 and NaOH. Write the equations for the preparation of phenol using these reagents.
The mechanism of hydration of ethene to form ethanol involves three steps.
Step 1:
Protonation of ethene to form carbocation by electrophilic attack of H3O+:

Step 2:
Nucleophilic attack of water on carbocation:
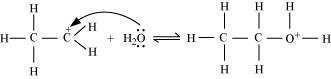
Step 3:
Deprotonation to form ethanol:


© 2026 GoodEd Technologies Pvt. Ltd.